CCNY research team in molecular breakthrough
Reducing a barrier that generally hinders the easy generation of new molecules, a team led by City College of New York chemist Mahesh K. Lakshman has devised a method to cleave generally inert bonds to allow the formation of new ones. The study is the cover story in the journal ACS Catalysis published by the American Chemical Society.
"Saturated carbon-hydrogen bonds in organic compounds are considered relatively inert and generally difficult to break in order to make other bonds, leading to new molecules," explained Lakshman, professor of chemistry in City College's Division of Science.
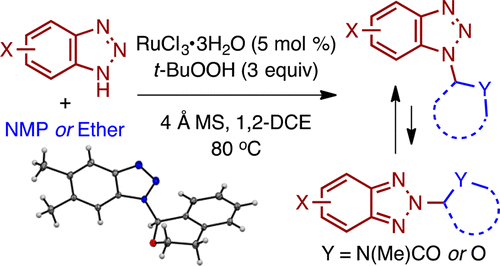
However, Lakshman and his colleagues demonstrated a method for accomplishing cleavage of carbon-hydrogen bonds and subsequent formation of carbon-nitrogen bonds.
Many of the ensuing new molecules bear structural similarities to the class of dideoxynucleosides, which are used as antiviral drugs. "Thus, this research can provide more direct access to novel pharmaceutical entities," said Lakshman, whose research thrust is organic synthesis at the chemistry-biology interface.
More information: Manish K. Singh et al. Ruthenium-Catalyzed C–H Bond Activation Approach to Azolyl Aminals and Hemiaminal Ethers, Mechanistic Evaluations, and Isomer Interconversion, ACS Catalysis (2016). DOI: 10.1021/acscatal.5b02603
Journal information: ACS Catalysis
Provided by City College of New York