December 18, 2015 weblog
Study clarifies the role of the leaving group in gas-phase bimolecular reaction
One of the first reaction mechanisms students learn in undergraduate organic chemistry is the SN2 reaction. Named because it involves the collision of two molecules, its energy barrier is related to the nucleophile, the solvent, and the leaving group. Exactly how the leaving group influences what is going on at the molecular level, however, remains elusive.
Using reaction cross-beam scattering and velocity map imaging combined with simulation studies a gas-phase SN2 reaction, F- + CH3Cl-, and comparing it to prior studies of F- + CH3I, Martin Stei, Eduardo Carrascosa, Martin A. Kainz, Aditya H. Kelkar, Jennifer Meyer, Istvan Szabo, Gabor Czako, and Roland Wester from the Institute for Ion Physics and Applied Physics, Universitat Innsbruck in Austria, the Institute of Chemistry, Eotvos University in Hungary, and the University of Szeged in Hungary have determined that the leaving group has a strong influence on reaction dynamics by influencing the orientation of the reactants. Specifically, they found that while both the chlorine and iodine reactions form a hydrogen bonding complex at the entrance channel, key differences cause one to be a direct rebound mechanism while the other is an indirect mechanism. Their work is reported in Nature Chemistry.
Reaction dynamics is the study of individual molecular collisions. While kinetic theory describes a mechanism based on aggregate behavior, reaction dynamics looks at individual particles. Reaction dynamics answers questions such as "Do the reactants need to collide in a certain way in order for a reaction to occur?" To answer questions about particular collisions, reaction dynamics studies must be conducted in the gas phase. This particular study looked at collisions using ion beams to control reactant velocity and TOF-MS to measure product velocity.
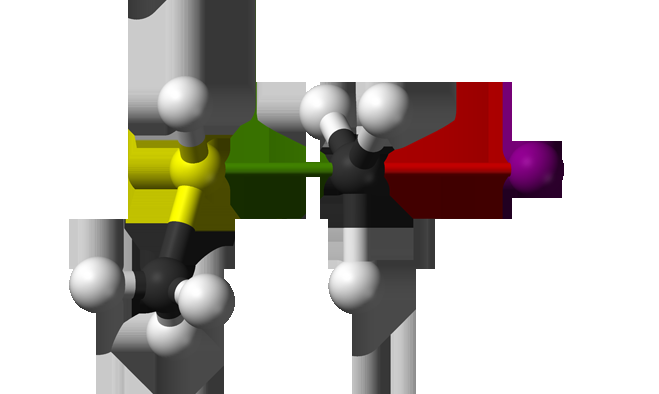
In the SN2 reaction F- + CH3Cl- à CH3F + Cl-, fluorine serves as the nucleophile, which engages in a "nucleophilic attack" on CH3Cl, and chlorine serves as the leaving group. This reaction occurs through an orchestrated process that, on the molecular level, shows that fluorine must collide directly with the electrophilic carbon, rather than the chlorine or hydrogens. Lower velocities did indicate an indirect complex-mediated mechanism, but for most velocities, this reaction undergoes a direct rebound mechanism. This is evidenced from scattering data that shows the chlorine leaving scattering in the backward direction. Lower velocities showed isotropic scattering. Even though there is evidence of a hydrogen bonding complex at the entrance channel, this reaction is not a slow, complex-dependent mechanism. The hydrogen complex, however, may affect reactant orientation.
This is not the case for the analogous reaction with iodine as a leaving group: F- + CH3I- à CH3F + I-. Prior studies showed that while this reaction does display some backscattering, it seems to have a greater isotropic distribution and higher internal energy at most velocities, indicating an indirect complex-mediated mechanism. For this reaction, the collinear entrance channel plays a more important role in lowering the reaction energy barrier than forming a hydrogen complex. In other words, the reactants may impact each other in various ways.
Simulation studies confirmed the experimental results of the chlorine reaction. Additional simulation studies showed that mass differences do not play a role in the differences between these reactions mechanisms, and impact parameter measurements indicate that reactant orientation is much more efficient in the case of the chlorine reaction compared to the iodine reaction. This is likely due to CH3Cl having a larger dipole moment compared to CH3I, which causes the fluorine ion to preferentially impact CH3Cl in a direct rebound orientation.
This study shows that given by comparing seemingly analogous gas-phase SN2 reactions, at the molecular level, their reaction dynamics are distinctly different. These differences are due to properties of the leaving group that are beyond differences in mass, and likely have to do with molecular orientation upon impact.
More information: Martin Stei et al, Influence of the leaving group on the dynamics of a gas-phase SN2 reaction, Nature Chemistry (2015). DOI: 10.1038/NCHEM.2400
Journal information: Nature Chemistry
© 2015 Phys.org