Tricking an enzyme into making better insulin

Mary Boyajian, a junior majoring in chemical engineering at Caltech, spent her summer as a student in the Summer Undergraduate Research Fellowships (SURF) program trying to trick an enzyme. The enzyme, tRNA synthetase, has a very specific chemical target, and Boyajian wanted the enzyme to ease up a bit on its requirements so that it might also find acceptable a slightly altered version of the target. The work might sound esoteric, but it was Boyajian's piece of a project with an end goal that could benefit millions: devising a faster-acting insulin-replacement therapy for the treatment of diabetes.
A normally functioning pancreas keeps blood sugar within a narrow range by releasing large bursts of the hormone insulin after meals. Insulin helps cells absorb excess glucose and prevents the liver from producing additional sugar. In the case of diabetics, however, either the cells become resistant to the effects of insulin or the body simply cannot produce enough of the hormone, so additional insulin is needed.
In the 1920s, insulin isolated from animals became the first insulin-replacement therapy for diabetics. Forty years later, scientists figured out how to make human insulin in the lab. However, that synthetic insulin behaves a bit differently in the body. For example, it tends to clump up and therefore takes a long time for the body to absorb.
To improve the speed or ease of absorption, chemists have designed replacement therapies that are analogs of human insulin, made by substituting some of insulin's building blocks, or amino acids, with other naturally occurring amino acids. However, there is room for improvement. For example, scientists would like to make therapies that kick in faster, last longer, and offer a longer shelf life.
In all current insulin-replacement therapies, certain naturally occurring amino acids are swapped for other naturally occurring amino acids. But in the lab of David Tirrell, the Ross McCollum–William H. Corcoran Professor and professor of chemistry and chemical engineering at Caltech, chemists are working with what are known as noncanonical amino acids. These variants are designed and made in the lab to have slightly altered chemical structures. If expressed in a protein, these synthetic amino acids can introduce entirely new functions or capabilities. Tirrell's group has the idea to swap out a naturally occurring amino acid from insulin with a noncanonical amino acid to create a replacement therapy that would outperform those on the market today.
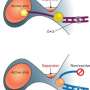
Boyajian's role this summer was to introduce specific mutations in the enzyme tRNA synthetase. Each of the 20 amino acids that are expressed naturally in proteins has its own tRNA synthetase that hunts within cells for its specific amino acid target, so that the amino acid can be incorporated in the right sequence to make proteins. Even a small difference in an amino acid's structure will deter its tRNA synthetase.
"When I started this project, I had no idea that changing one amino acid could change so much about a protein, but it can," says Boyajian. "My job is to mutate the tRNA synthetase so that it won't see a modified amino acid—one of our noncanonical amino acids—and say, 'That's the wrong one. Take it out.'"
To get an idea of how she might mutate the enzyme, Boyajian studied the known structures of similar tRNA synthetases and how they interact with their target molecules.
Once she had an idea for a mutation, she introduced the changes into the gene that codes for the tRNA synthetase. Then she used a standard technique in molecular biology called polymerase chain reaction (PCR) to make many copies of it. Next she grew cells with the mutated enzymes on media lacking the naturally occurring amino acid—think of it as a type of food for cells. Once the cells ate up any small traces of the amino acid in the media, she fed them one of the noncanonical amino acids. If a mutated enzyme worked, it was able to "eat" the new amino acids; if not, the cells eventually died.
At the end of the summer, one of Boyajian's mutated tRNA synthetases showed promising results in terms of incorporating one of the noncanonical amino acids, and she is now working to scale-up the size of cultures to determine whether the new enzyme can be used to produce proteins for future experiments. In the long term, if the enzyme is found to efficiently incorporate a specific noncanonical amino acid, the Tirrell lab would use the enzyme to produce novel insulins that could be assessed as potential biopharmaceuticals to improve the quality of life for patients.
Boyajian, who also plays basketball and serves as one of the captains of the water polo team, says she learned a lot from her SURF experience. "My grad student mentors, Seth Lieblich and Kat Fang, were great, and everybody in the lab was very welcoming," she says. "It's really nice to see everything you learned in the classroom being applied."
Provided by California Institute of Technology