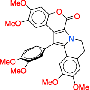
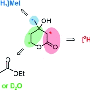Bengamide antitumor agent displaying antiproliferative potency
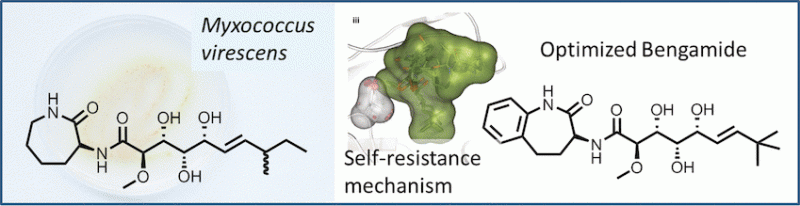
Scientists from the Helmholtz Centre for Infection Research in Germany have identified a terrestrial myxobacterium as a promising source for the bengamide class of natural products, which were originally thought to be secondary metabolites of marine sponges only. As reported in the journal Angewandte Chemie, the derivatives of the bengamides, which now can be expressed on a large scale, display great potential as anticancer drugs, with unleveraged potential in other indications, such as obesity.
Microorganism living in the soil are expected to contain a plethora of potentially cytotoxic compounds, but it came also as a surprise when scientists discovered that terrestrial myxobacteria also produced bengamides, a class of potential antitumor agents that were originally known only from marine sponges. In a multidisciplinary approach, Rolf Müller and Mark Brönstrup at the Helmholtz Centre in Germany and researchers from Sanofi in Paris and Frankfurt have now extensively investigated the myxobacterial biosynthetic pathway of the bengamides and also explored their synthetic and semisynthetic derivatives for their pharmacokinetic properties.
The key to success was fermentation, as the authors say: "The gram-scale fermentative access to this class of "marine" natural products allowed studies to be conducted on the biosynthesis of bengamides, their heterologous expression, and the self-resistance mechanism of their producer. We also optimized their properties as drug leads by semi- and total synthesis". The molecular target of the bengamides is known to be the humane methionine aminopeptidases (MetAP). By exploring the expression systems of the bacteria, the scientists could specify one single amino acid in the bacterial MetAP that provides their self-resistance against the bengamide inhibitors. As the natural bengamide derivatives were found to degrade relatively fast by microsomal activity, the scientists chemically modified the bengamide skeleton to obtain more stable compounds. "A semisynthetic access to such analogues is conceivable by combining a microbially produced polyketide side chain with synthetic caprolactams," the authors state, and although in this case they chose total synthesis for enhancing the potency and stability of the bengamides, for future research they expect a combination of genetic engineering and synthetic approaches to be a feasible and cost-effective way to produce optimized bengamide drugs.
The authors also remark that MetAP2 inhibition by bergamides could be further explored as a means of increasing fat metabolism and reduction of body weight. Although the bengamides were originally assigned to marine sources, the natural compounds as well as their derivatives are now available from terrestrial sources by genetic engineering. Indeed the engineered compounds may represent drug leads to be extensively explored.
More information: Silke C. Wenzel et al. Production of the Bengamide Class of Marine Natural Products in Myxobacteria: Biosynthesis and Structure-Activity Relationships, Angewandte Chemie International Edition (2015). DOI: 10.1002/anie.201508277
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Angewandte Chemie