New high-performance electrode material for sodium full cells
The requirements for batteries used in stationary energy storage are nothing less than long lifecycle, low cost, high safety, high efficiency, and high operating voltage. Replacing lithium with sodium would be an option to reduce costs massively, and in the journal Angewandte Chemie, Chinese and Japanese scientists report the development of a high-performance sodium fuel cell based on a bipolar, sodium-containing electrode material, which exhibits very promising characteristics towards large-scale applications.
Renewable energies with their unpredictable availability and locational dependence make the establishment of smart grids and high-performance battery systems urgent, but in particular, the battery technology is still far from perfect. To make the technology cheaper, sodium has been suggested as replacement for lithium. However, the sodium ion is larger and so the electrode materials must be able to resist the volume change associated with the charging and discharging process. In addition, the anode material has to cope with a more problematic oxidation potential of sodium, which makes the search for a convenient cell extremely complicated. The amazing solution that Haoshen Zhou and his colleagues at the National Institute of Advanced Industrial Science and Technology (AIST) and Tohoku University in Japan and Nanjing University in China present involves employing the same electrode material for anode and cathode in a completely symmetric cell.
The material developed is a titanium mixed oxide, which the scientists called P2-NNCT for the P2 phase of Na0.66Ni0.17Co0.17Ti0.66O2, where sodium, nickel, cobalt, and titanium have nonequal amounts. As the scientists have expected, the nickel and cobalt centers have sufficient redox properties to serve as the cathode, while the titanium oxide represents the anode side. Of pivotal importance is, however, the layered rigid structure of the material with the sodium ions clamped between the metal oxide sheets but in a way that allows the ions to travel freely in the charging and discharging processes.
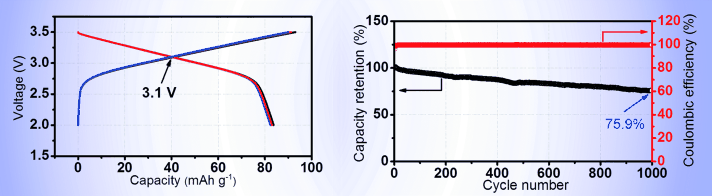
Assembled as a full cell, "the new material exhibited the highest average voltage of 3.1 V in reported sodium symmetric full cells and the longest cycle life of 1000 cycles in all reported sodium cells," Zhou says. With the Coulombic efficiency of the whole cycling process being close to 99.9%, except for the initial cycles, it is thus suitable to serve as an energy storage device in practical application. Therefore the authors state very clearly: "Our optimized sodium symmetric cells based on P2-NNCT outperform all other sodium full cells." Moreover, the safety concerns about the discharging at the anode, which had impeded the further development of sodium batteries, now seem to be solved as well. All this sounds like a very promising advance towards advanced battery technologies.
More information: "A High-Voltage and Ultralong-Life Sodium Full Cell for Stationary Energy Storage." Angew. Chem. Int. Ed.. doi: 10.1002/anie.201505215
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Wiley