Uncovering the mechanism of photoluminescence stabilization in semiconductor nanoparticles
A collaborative of Korean researchers has discovered the mechanism of photoluminescence (PL) loss from semiconductor nanoparticles called quantum dots (QDs) and proposed an effective method for stabilization of PL.
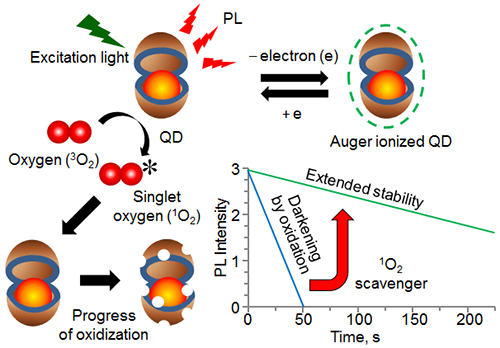
In the present study, the researchers tethered QDs sparsely on the surface of a cover glass and observed the PL from single QDs in an optical microscope. They discovered that the release of an electron (Auger ionization) from photo-excited QDs prevents oxidation of QDs by singlet oxygen (1O2), leading to PL stabilization. In addition, they found that scavengers of 1O2 stabilize the PL of QDs in the neutral state without Auger ionization. These findings are expected to make substantial impacts on single molecule bio-imaging, a technique for studying how individual molecules work in living cells.
Details of this discovery are published in Angewandte Chemie International Edition in English and Angewandte Chemie in German, journals by the German Chemical Society, on March 23, 2015.
If bio-molecules, such as nucleic acids and proteins, can be detected and observed one by one in living cells, it will be possible to develop medicines and diagnose diseases efficiently and precisely. Recently, such a detection called single molecule bio-imaging is becoming possible; thanks to the development of ultra-high-sensitive microscopes. Nonetheless, there are still unsolved problems in single molecule bio-imaging. Since bio-molecules such as nucleic acids and proteins in their native forms cannot be observed at high sensitivity using optical microscopes, these molecules are chemically modified with fluorescent dyes. However, conventional organic dyes suffer from quick photo-darkening, which makes such dyes less attractive in prolonged single molecule bio-imaging. Although QDs are superior to organic dyes in photo-stability, their PL is inevitably diminished when exposed to light for a long time.
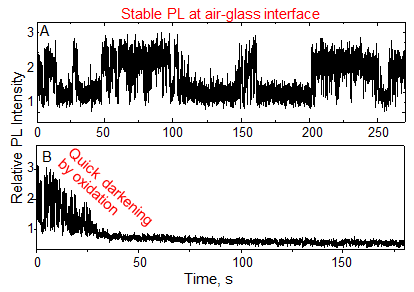
PL intensity of single QDs gradually decreases and eventually disappears when irradiated with light for a long time. This behavior is quite different from the one-step photo-darkening of single molecules of organic dyes. The gradual decrease of PL intensity is attributed to reactions of QDs with oxygen and the conversion of QDs into non-luminescent or poorly luminescent oxidized species. Thus, considerable efforts have been made globally for preventing photo-oxidation and obtaining stable PL from QDs, though such attempts have never been successful.
AIST has been working to develop novel photo-luminescent nanomaterials as well as technologies for stabilizing the PL of QDs. In this study, in collaboration with Kagawa University and Nagaoka University of Technology, AIST investigated the production of 1O2 by QDs and oxidation of QDs by 1O2, which led to the present discovery.
This research was supported by Japan Society for the Promotion of Science under the Grant-in-Aid for Scientific Research program to Young Scientists (B) and Japan Science and Technology Agency under the Precursory Research for Embryonic Science and Technology (PRESTO) program.
Samples of QDs were prepared by the chemical tethering of CdSe/ZnS QDs on the surface of glass cover slips at a uniform density of about 100 QDs per 100×100 µm2 area. The intensity of PL (Fig. 1A) from single QDs was observed on an optical microscope under 532 nm laser excitation. The high-speed fluctuation or ON-OFF behavior of PL is called "blinking," a characteristic phenomenon of PL from single QDs. Blinking is known to be caused by Auger ionization. The OFF periods often continue for a few seconds to several tens of seconds. Despite this blinking behavior, the PL intensity of single QDs on glass cover slips was stable in air for an extended period of time. On the other hand, when single QDs on glass cover slips were immersed in an organic solvent, namely dimethylsulfoxide (DMSO), the PL intensity quickly diminishes and eventually disappears (Fig. 1B). Similar PL loss was observed when single QDs were immersed in water.
When QDs are immersed in DMSO or water, the excited QDs efficiently transfer energy to dissolved oxygen and produce 1O2, and the QDs themselves are oxidized. When such a reaction repeatedly occurs, non-luminescent oxide islands are formed on the surface of QDs, which induces the gradual loss of the QD's PL. The production of 1O2 was confirmed from observation of the characteristic phosphorescence (about 1270 nm) of 1O2. On the other hand, the production of 1O2 and oxidation of QDs by 1O2 are inefficient in air due to the heterogeneous nature of the air-QD interface, which resulted in the observation of stable PL.
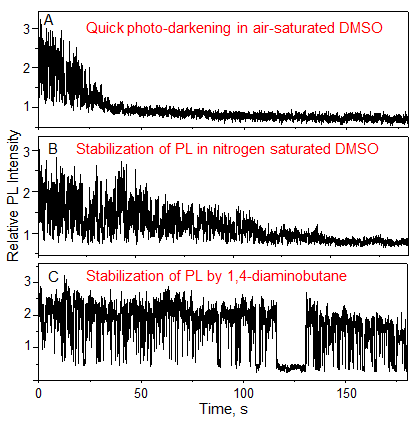
The PL intensity from single QDs quickly diminishes in air-saturated DMSO (Fig. 2A); whereas, the loss of PL slows down in DMSO saturated with nitrogen (Fig. 2B). Further, stable PL emission was observed from single QDs in DMSO saturated with air and supplemented with 1,4-diaminobutane as a 1O2 scavenger, which is a chemical that rapidly reacts with 1O2 (Fig. 2C). These results indicate that 1O2 prevents stable PL emission from single QDs.
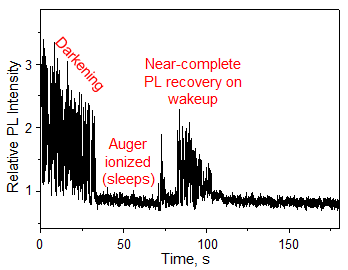
The single QDs irradiated with laser light suffer from intrinsic ON and OFF PL, which is caused by Auger ionization. However, the PL intensity after each OFF period recovers to almost the same level as that before the OFF period (Fig. 3). While QDs exist in the Auger ionized state during the OFF period, PL does not decrease. In other words, the Auger ionized QDs do not undergo oxidation.
In order to obtain incessant PL from various nanomaterials, future research will aim at other nanomaterials for systematically revealing relations among charge carrier relaxations, Auger ionization, surface defects, production of reactive oxygen, and oxidation. Also, the researchers will consider formulation of nanobioconjugates made of semiconductors, organic and biological materials, etc. for uninterrupted PL-based bio-imaging at single-molecule levels.
Journal information: Angewandte Chemie , Angewandte Chemie International Edition