New transitory form of silica observed
A Carnegie-led team was able to discover five new forms of silica under extreme pressures at room temperature. Their findings are published by Nature Communications.
Silicon dioxide, commonly called silica, is one of the most-abundant natural compounds and a major component of the Earth's crust and mantle. It is well-known even to non-scientists in its quartz crystalline form, which is a major component of sand in many places. It is used in the manufacture of microchips, cement, glass, and even some toothpaste.
Silica's various high-pressure forms make it an often-used study subject for scientists interested in the transition between different chemical phases under extreme conditions, such as those mimicking the deep Earth.
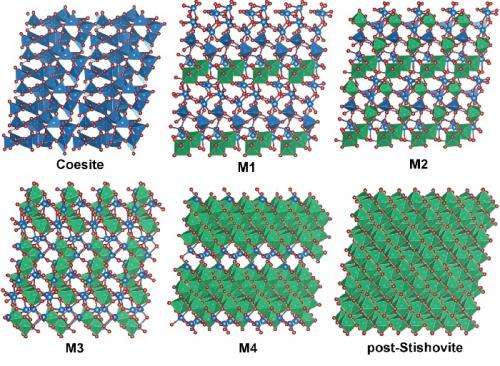
The first-discovered high-pressure, high-temperature denser form, or phase, of silica is called coesite, which, like quartz, consists of building blocks of silicon atoms surrounded by four oxygen atoms. Under greater pressures and temperatures, it transforms into an even denser form called stishovite, with silicon atoms surrounded by six oxygen atoms. The transition between these phases was crucial for learning about the pressure gradient of the deep Earth and the four-to-six configuration shift has been of great interest to geoscientists. Experiments have revealed even higher-pressure phases of silica beyond these two, sometimes called post-stishovite.
A chemical phase is a distinctive and uniform configuration of the molecules that make up a substance. Changes in external conditions, such as temperature and pressure, can induce a transition from one phase to another, not unlike water freezing into ice or boiling into steam.
The team, including Carnegie's Qingyang Hu, Jinfu Shu, Yue Meng, Wenge Yang, and Ho-Kwang, "Dave" Mao, demonstrated that under a range from 257,000 to 523,000 times normal atmospheric pressure (26 to 53 gigapascals), a single crystal of coesite transforms into four new, co-existing crystalline phases before finally recombining into a single phase that is denser than stishovite, sometimes called post-stishovite, which is the team's fifth newly discovered phase. This transition takes place at room temperature, rather than the extreme temperatures found deep in the earth.
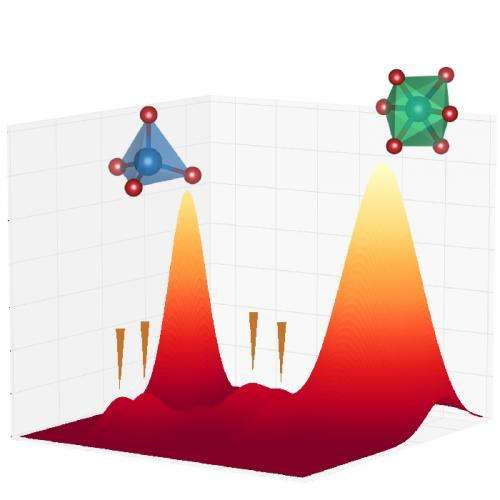
Scientists previously thought that this intermediate was amorphous, meaning that it lacked the long-range order of a crystalline structure. This new study uses superior x-ray analytical probes to show otherwise—they are four, distinct, well-crystalized phases of silica without amorphization. Advanced theoretical calculations performed by the team provided detailed explanations of the transition paths from coesite to the four crystalline phases to post-stishovite.
"Scientists have long debated whether a phase exists between the four- and six-oxygen phases," Mao said. "These newly discovered four transition phases and the new phase of post-stishovite we discovered show the missing link for which we've been searching."
Journal information: Nature Communications
Provided by Carnegie Institution for Science