Earth's lower mantle chemistry breakthrough
Breaking research news from a team of scientists led by Carnegie's Ho-kwang "Dave" Mao reveals that the composition of the Earth's lower mantle may be significantly different than previously thought. These results are to be published by Science.
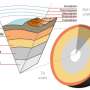
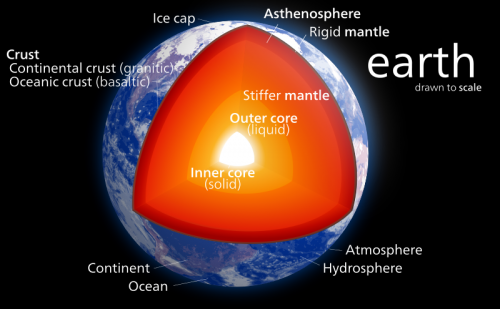
The lower mantle comprises 55 percent of the planet by volume and extends from 670 and 2900 kilometers in depth, as defined by the so-called transition zone (top) and the core-mantle boundary (below). Pressures in the lower mantle start at 237,000 times atmospheric pressure (24 gigapascals) and reach 1.3 million times atmospheric pressure (136 gigapascals) at the core-mantle boundary.
The prevailing theory has been that the majority of the lower mantle is made up of a single ferromagnesian silicate mineral, commonly called perovskite (Mg,Fe)SiO3) defined through its chemistry and structure. It was thought that perovskite didn't change structure over the enormous range of pressures and temperatures spanning the lower mantle.
Recent experiments that simulate the conditions of the lower mantle using laser-heated diamond anvil cells, at pressures between 938,000 and 997,000 times atmospheric pressure (95 and 101 gigapascals) and temperatures between 3,500 and 3,860 degrees Fahrenheit (2,200 and 2,400 Kelvin), now reveal that iron bearing perovskite is, in fact, unstable in the lower mantle.
The team finds that the mineral disassociates into two phases one a magnesium silicate perovskite missing iron, which is represented by the Fe portion of the chemical formula, and a new mineral, that is iron-rich and hexagonal in structure, called the H-phase. Experiments confirm that this iron-rich H-phase is more stable than iron bearing perovskite, much to everyone's surprise. This means it is likely a prevalent and previously unknown species in the lower mantle. This may change our understanding of the deep Earth.
"We still don't fully understand the chemistry of the H-phase," said lead author Li Zhang, also of Carnegie. "But this finding indicates that all geodynamic models need to be reconsidered to take the H-phase into account. And there could be even more unidentified phases down there in the lower mantle as well, waiting to be identified."
More information: "Disproportionation of (Mg,Fe)SiO3 perovskite in Earth's deep lower mantle," by L. Zhang et al . Science, 2014. www.sciencemag.org/lookup/doi/ … 1126/science.1250274
Related: New insight into the temperature of deep Earth: phys.org/news/2014-05-insight- … ture-deep-earth.html
Journal information: Science
Provided by Carnegie Institution for Science