Understanding nickel catalysis
Catalysis is a chemical phenomenon that increases the rate of a chemical reaction by spending only a tiny amount of additional substance, called a catalyst. Around 90 percent of all commercially manufactured products involve chemical substances processed in catalytic procedures at some stage. Whatever we are surrounded by in our everyday life was produced with the help of chemical catalysis – materials, dyes, textile, fuels, paper, devices, vehicle parts, food, pharmaceuticals, cosmetics and many others.
Since only a small amount of a catalyst is required to drive the production of chemical substances, the catalytic process can be tuned to high cost efficiency. Polymerization is an excellent representative example of catalytic efficiency. The manufactured products – polyethylene, plastics, etc. – are produced at a million tons scale every year and are consumed in all countries. Preparation of pharmaceuticals and drugs is another example. Being produced at much smaller scale, these unique chemicals require exceptional structural diversity. It is the area where an outstanding potential of catalysis is now comprehensively explored.
Such increasing growth of industrial production requires the development of more and more catalysts. The price of metal catalysts has significantly increased and even tiny amounts have become expensive. For example, the Nasdaq price for one widely used catalytic metal, palladium, increased by more then four times during the last six years. Production of catalysts is the main area of consumption of palladium with many demanding applications.
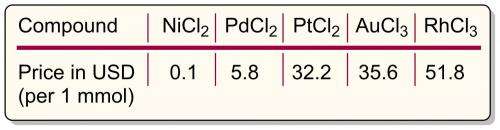
Today, the tendency is clearly shifting toward replacement of expensive metals by cheaper analogs. A brief comparison of catalyst prices shows that a simple metal like nickel is superior to Pd, Pt, Au, Rh (Figure 1). At the moment, these noble metals are ubiquitously used in catalytic applications.
So is it possible to replace noble metals with nickel in the catalytic applications? This appears to be rather difficult to achieve. Each metal has a unique set of chemical and physical properties that provide a particular catalytic transformation. Replacing one catalytic metal for another is in many cases a challenging task.
Of particular interest is the observation made about nickel a century ago by the famous chemist and Nobel Laureate Paul Sabatier: "It can be compared to a spirited horse, delicate, difficult to control, and incapable of sustainable work." (Sabatier, P. Catalysis in Organic Chemistry, NY, 1922, p. 15.). Thus, nickel catalysis is a long-standing question. Can the chemists force nickel to do a desired synthetic work? Although there are many successful nickel catalysts on the market, researchers are still facing the challenge.
After multiple unsuccessful direct forcing attempts, chemists have made impressive progress in understanding the mechanisms of nickel-catalyzed reactions. New research provides the opportunity to understand the "spirit" of the metal and to make use of its advantages.
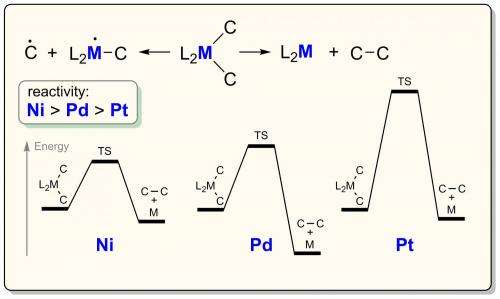
A recent article published in the ACS Catalysis journal highlights state-of-the-art in this field and emphasizes that we have reached a turning point in the studies of nickel catalysis. As it is discussed in the article, fundamental knowledge on reaction mechanisms can fully uncover the power of nickel catalysts and minimize plausible drawbacks for practical applications (Figure 2). The key advantages of nickel catalysts, summarized in the article, include:
- High performance in reactions for which other metals were not efficient;
- Large variability of electronic states – Ni(0)/Ni(I)/Ni(II)/Ni(III);
- New reactions and transformation beyond the known limits of other metals;
- Facile activation and transformation of molecules that are chemically less reactive;
- Excellent opportunities in photocatalytic and hybrid catalytic cycles.
Will a new hero appear soon on the catalysis team? Let's give it some time.
More information: Valentine P. Ananikov, "Nickel: The 'Spirited Horse. of Transition Metal Catalysis", ACS Catal., 2015, 5, pp. 1964 – 1971. DOI: 10.1021/acscatal.5b00072
Journal information: ACS Catalysis
Provided by Zelinsky Institute of Organic Chemistry of Russian Academy of Sciences





















