Controlling car pollution at the quantum level

Toyota Central R&D Labs. in Japan have reviewed research that might be leading the way towards a new generation of automotive catalytic converters.
Catalytic converters that change the toxic fumes of automobile exhaust to less toxic pollutants only reached the market in the mid-1970s. They are formed of a catalyst – usually in the form of a precious metal such as platinum, palladium, or rhodium – a catalyst support material, and a wash-coat designed to disperse the catalytic materials over a wide surface area.
Toyota Central R&D Labs. Inc. in Japan are involved in research to develop catalysts that are controlled at the quantum-level. With this level of control, "we can expect an extreme reduction of precious metal usage in automotive exhaust catalysts and/or fuel cells," says Dr. Yoshihide Watanabe, chief researcher at the Toyota Central R&D Labs in Japan.
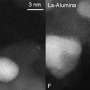
He reviewed research on different types of catalytic reactions involving metal clusters whose sizes were atomically controlled.
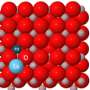
Metal cluster chemistry has been developing rapidly since the mid-20th century. A cluster is a group of atoms or molecules formed by interactions varying in strength from very weak to strong. Some naturally occurring clusters are known to be involved in catalytic reactions. The study of metal clusters is inspiring great interest, partially for the potential use of synthetic clusters in industrial applications, such as catalysts in catalytic converters.
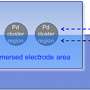
He pointed out that not much research has been done in the area of atomically controlled cluster catalysis, with the exception of studies on carbon monoxide oxidation reaction.
His research indicates that catalytic activity is strongly affected by the electronic structure of clusters, their geometry on a support material, and the interaction between the cluster and the material. Thus, the catalytic activity of clusters can be enhanced by controlling cluster size and the interaction between the clusters and the support material. This is important, because enhancing the catalytic activity of some clusters may greatly reduce the utilization of precious metals as catalytic agents. A few studies that try to understand how the catalytic properties of size-controlled clusters are affected at the quantum level. Although several mechanisms for these effects are suggested, the field is still in progress, he says.
As a result of his review, Watanabe recommends further studies that investigate how catalytic reaction rates are affected by temperature. He says that applying computer simulations, known as computational chemistry, can lead the way towards developing quantum-controlled catalysts formed from atomically precise clusters.
More information: "Atomically precise cluster catalysis towards quantum controlled catalysts." Watanabe 2014 Sci. Technol. Adv. Mater. 15 (2014) 063501 (12pp). DOI: 10.1088/1468-6996/15/6/063501, iopscience.iop.org/1468-6996/15/6/063501
Provided by National Institute for Materials Science