Chemists develop 'nanoreactor' for discovering new chemical reactions
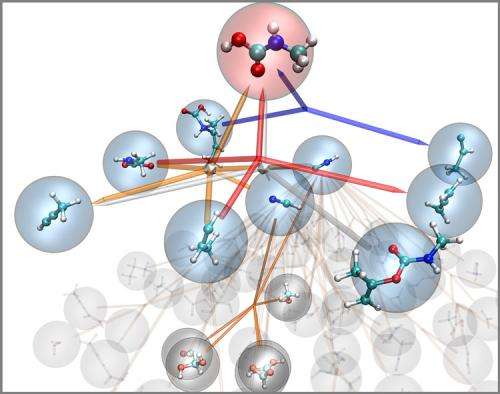
(Phys.org) —In 1952, the famous Urey-Miller experiment mixed together chemicals that were present early in Earth's history, then approximately replicated the environmental conditions on the planet at that time to see if biologically relevant organic molecules would form spontaneously.
That experiment produced more than 20 molecules that are important to life, but a team of Stanford chemists thinks it can do one step better.
The group has built a computer model that can not only determine all the possible products of the Urey-Miller experiment, but also detail all the possible chemical reactions that lead to their formation. The nanoreactor, as they call the model, could help scientists discover chemical reactions and mechanisms that improve the efficiency of fuel combustion or batteries, or reveal opportunities for new drugs.
The nanoreactor, which was described in a recent issue of Nature Chemistry, works something like a virtual chemistry set. Simply enter the structure of some target chemicals into the computer model, set the environmental conditions – such as temperature or pressure – and let it run.
Then, algorithms begin to solve the quantum mechanical problems for each electron in the molecules as they interact – where are they likely to move from chemical to chemical, and what mechanisms must occur for those movements to take place? Each step is recorded along the way.
"You can just hit a button and it will tell you all the reactions that are important," said senior author Todd Martinez, the David Mulvane Ehrsam and Edward Curtis Franklin Professor of Chemistry at Stanford. "It uses a hybrid approach that incorporates physics and machine learning to discover all the possible ways that your chemicals might react, and that might include reactions or mechanisms we've never seen before."
Traditionally, producing this type of information involves sitting down with a pencil and paper and sketching electron movements, which limits the work to sets of a few atoms because of the sheer complexity and number of possible outcomes. Running on a desktop computer, the nanoreactor can simulate 100 to 200 atoms at a time, and produce results in a couple of hours.
The nanoreactor could reveal reactions and mechanism that have tremendous applications in refining important chemical processes that we rely on every day. Consider, for instance, combustion reaction that powers gas-fueled automobiles.
"Combustion involves many hundreds of reactions, and we don't even know all that's occurring. This is a way to discover those using theory," Martinez said. "If you can know all the reactions, then you can identify which are actually key to sustaining combustion, and which lead to detrimental soot. And then you could maybe figure out how to stop soot formation, or to shut down other undesirable reaction pathways."
Martinez expects the nanoreactor to reveal opportunities for developing catalysts that improve the efficiency of known reactions, particularly in applications such as fuel cells or batteries. Similarly, the model could provide a better understanding of the biochemical reactions critical in human health and disease, leading the way to new drug development.
More information: "Discovering chemistry with an ab initio nanoreactor." Nature Chemistry (2014) DOI: 10.1038/nchem.2099
Journal information: Nature Chemistry
Provided by Stanford University



















