October 27, 2014 feature
Water, water everywhere: How UV irradiation reversibly switches graphene between hydrophobic and hydrophilic states
(Phys.org) —Scientists have long observed that the wettability of graphene – an essentially two-dimensional crystalline allotrope of carbon that it interacts oddly with light and with other materials – can be reversed between hydrophobic and hydrophilic states by applying ultraviolet (UV) irradiation. However, an explanation for this behavior has remained elusive. Recently, researchers at The University of New South Wales and University of Technology, Sydney investigating this phenomenon both experimentally and by calculations using density functional theory (DFT) – a computational quantum mechanical modeling method – finding that UV irradiation enables this reversible and controllable transition in graphene films having induced defects by water splitting adsorption on the graphene surface of H2O molecules in air. (Water splitting is the chemically dissociative reaction in which water is separated into hydroxyl and hydrogen; hydroxyl is a chemical functional group containing an oxygen atom connected by a covalent bond to a hydrogen atom; and adsorption is the adhesion of atoms, ions, or molecules from a gas, liquid, or dissolved solid to a surface.)
The scientists conclude that their discovery may provide new insights into the fundamental principles of water splitting with graphene-based materials, and could thereby lead to other applications – including electrocatalysis, nanomaterials; nanoelectromechanical systems, biomaterials, microfluidic devices, hybrid organic systems, and other advanced multifunctional systems.
Dr. Zhimin Ao discussed the paper that he, Doctoral Student Zhemi Xu and their co-authors published in Scientific Reports and the main challenges the researchers faced. "The main challenge – and the motivation for the conducting the study – was to reveal the real mechanism of the reversible wettability transition under UV irradiation and isolate it from various possible reasons, such as the contamination of chemicals on samples or induced by molecules in air," Ao tells Phys.org. "We also had to identify H2O rather than other possible molecules in air, which contributes the wettability transition under UV irradiation." After determining the contribution of H2O, he adds, another challenge was to understand the adsorption type of H2O for the wettability transition – that is, chemical or physical adsorption.
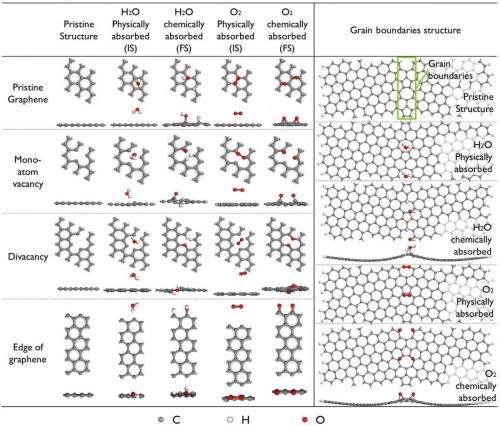
"Secondly," Ao continues, "to eliminate drawbacks from chemical doping and induced defects – such as organic molecules on the graphene sample – that may be an important factor in graphene's wettability transition under UV, the samples were stored for two hours in a vacuum to remove contaminants on the graphene surface." As a result, most of the remaining graphene defects, such as vacancies, edges and grain boundary, would be there due to the synthesis process.
"According to our calculations, on defects of vacancies, edge and grain boundary, water splitting can be easier to achieve. However, other defects can also affect the wettability of graphene, such as aluminum doping, which has been reported by another paper1 of my group."
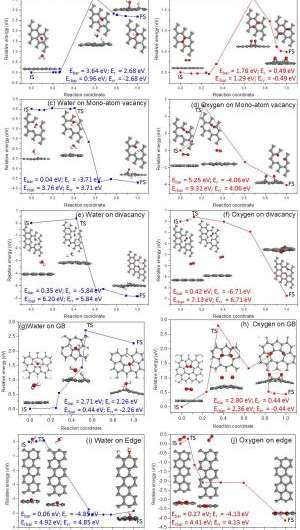
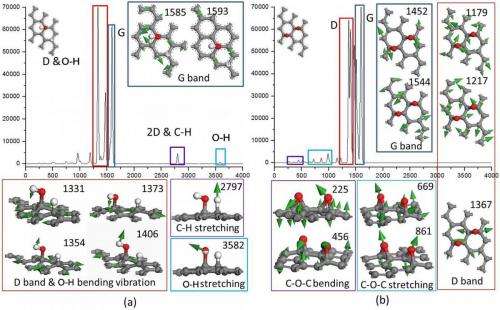
The key technique the researchers used to address these challenges was to combine experiment and first-principles calculations. "In our experiment, we demonstrated that the wettability of graphene could be reversibly tuned through UV irradiation in air and vacuum storage," Ao says. "In addition, computational calculations enable us to understand the exact effect of each individual factor." After comparing their experimental and calculation results, the scientists found that Raman spectra from the experiment were similar to that of H2O dissociative adsorption on graphene. (In graphene research, Raman spectroscopy is used to determine the number and orientation of layers, the quality and types of edge, and the effects of perturbations, such as electric and magnetic fields, strain, and doping.) Moreover, they also considered irradiations at different conditions, such as in O2 and H2O rich environments, and found that H2O concentration clearly affected the wettability change of graphene after irradiation. "Therefore," Ao adds, "we concluded that H2O dissociative adsorption on graphene induces the reversible wettability transition."
The direct application for this approach is water splitting – a very important step in, for example, hydrogen generation: Using the technique in this work, H2O molecules could be easily split into OH- and H+ groups and adsorbed on defect-induced graphene under UV irradiation. After irradiation, the two groups can be easily desorbed from the graphene and produce hydrogen, allowing the graphene to be used continually as a catalyst for water splitting.
Ao points out that when fabricating devices based on graphene – for example, solar cells – layer-by-layer materials fabrication is required. "Hydrophilic graphene is more easily modified and combined with other materials than is hydrophobic graphene. For example, in the case of biomaterials, hydrophilic graphene would be desirable for the biomolecule contact."
It turns out that achieving graphene reversible wettability can be accomplished using other techniques, including external electric fields, plasma treatment, magnetic fields, and neutron diffraction. "Actually, the work with achieving graphene reversible wettability using external electric fields was also reported2 by my group based on first-principle calculations. Compared with using external electric fields, UV irradiation is easily realized in experiment, while a very high electric field is required to realize the wettability transition," noting that an experiment under a strong electric field is underway. "Plasma has even greater energy, and may induce more defects in graphene. However, the plasma treatment process is more complicated and has greater requirements."
Looking ahead, Ao notes that they need to further clarify the mechanism for graphene's hydrophobic to hydrophilic transition under UV irradiation because the latter itself can induce graphene defects. "Although UV irradiation was believed to induce defects in graphene, the problem is that these defects aren't obvious because this energy source is not strong enough. To further clarify the reversible wettability mechanism, we may use different energy sources to investigate the transition, such as X-ray and neutron diffraction." They also plan to investigate conductivity change and transport properties under UV irradiation.
"High electrical conductivity graphene film with high hydrophilicity is always desirable," Ao tells Phys.org. "However, these two properties are normally resisting each other. When working with graphene-based devices, exploring the electric conductivity variation of graphene in such processes can help to control and balance these two properties."
Other areas that might benefit from their study, Ao concludes, include sensors and hydrogen generation and storage.
More information: Reversible Hydrophobic to Hydrophilic Transition in Graphene via Water Splitting Induced by UV Irradiation, Scientific Reports (Published online September 23 2014), 4:6450, doi:10.1038/srep06450
Related:
1First principles study on the hydrophilic and conductive graphene doped with Al atoms, Physical Chemistry Chemical Physics, 2013, 15, 10859-10865, doi:10.1039/C3CP00128H
2Reversible Transition of Graphene from Hydrophobic to Hydrophilic in the Presence of an Electric Field, Journal of Physical Chemistry C, 2012, 116 (36), doi:10.1021/jp3050466
Journal information: Scientific Reports , Physical Chemistry Chemical Physics , Journal of Physical Chemistry C
© 2014 Phys.org