Research unlocks potential of super-compound
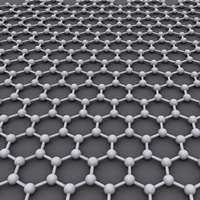
Researchers at The University of Western Australia's have discovered that nano-sized fragments of graphene - sheets of pure carbon - can speed up the rate of chemical reactions.
Assistant Professor Amir Karton, from UWA's School of Chemistry and Biochemistry, said the finding, published this week in Chemical Physics Letters journal, was significant because it suggested that graphene might have potential applications in catalysing chemical reactions of industrial importance.
Graphene was one of the most exciting materials to work with in nanotechnology because its two-dimensional structure and unique chemical properties made it a promising candidate for new applications such as energy storage, material composites as well as computing and electronics, Assistant Professor Karton said.
"Ever since the discovery of graphene in 2004, scientists have been looking for potential applications in nanochemistry," he said.
"Using powerful supercomputers, researchers at UWA discovered that graphene nanoflakes can significantly enhance the rates of a range of chemical reactions."
Graphene is remarkably strong for its low weight - about 100 times stronger than steel - and it conducts heat and electricity with great efficiency. The global market for graphene is reported to have reached US$9 million this year with most sales concentrated in the semiconductor, electronics, battery energy and composites.
Assistant Professor Karton said the current investigation showed that graphene nonoflakes could efficiently catalyse a range of chemical reactions.
"The next steps would be to extend the catalytic scope to other types of chemical reactions and extend the scope of the study to 'infinite' graphene sheets rather than graphene nanoflakes," he said.
More information: Amir Karton, "Inversion and rotation processes involving non-planar aromatic compounds catalyzed by extended polycyclic aromatic hydrocarbons," Chemical Physics Letters, Volume 614, 20 October 2014, Pages 156-161, ISSN 0009-2614, dx.doi.org/10.1016/j.cplett.2014.09.032.
Provided by University of Western Australia