Harvesting hydrogen fuel from the Sun using Earth-abundant materials

The race is on to optimize solar energy's performance. More efficient silicon photovoltaic panels, dye-sensitized solar cells, concentrated cells and thermodynamic solar plants all pursue the same goal: to produce a maximum amount of electrons from sunlight. Those electrons can then be converted into electricity to turn on lights and power your refrigerator.
At the Laboratory of Photonics and Interfaces at EPFL, led by Michael Grätzel, where scientists invented dye solar cells that mimic photosynthesis in plants, they have also developed methods for generating fuels such as hydrogen through solar water splitting.
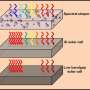
To do this, they either use photoelectrochemical cells that directly split water into hydrogen and oxygen when exposed to sunlight, or they combine electricity-generating cells with an electrolyzer that separates the water molecules.
By using the latter technique, Grätzel's post-doctoral student Jingshan Luo and his colleagues were able to obtain a performance so spectacular that their achievement is being published today in the journal Science. Their device converts into hydrogen 12.3 percent of the energy diffused by the sun on perovskite absorbers – a compound that can be obtained in the laboratory from common materials, such as those used in conventional car batteries, eliminating the need for rare-earth metals in the production of usable hydrogen fuel.
Bottled sun
This high efficiency provides stiff competition for other techniques used to convert solar energy. But this method has several advantages over others:
"Both the perovskite used in the cells and the nickel and iron catalysts making up the electrodes require resources that are abundant on Earth and that are also cheap," explained Jingshan Luo. "However, our electrodes work just as well as the expensive platinum-based models customarily used."
On the other hand, the conversion of solar energy into hydrogen makes its storage possible, which addresses one of the biggest disadvantages faced by renewable electricity—the requirement to use it at the time it is produced.
"Once you have hydrogen, you store it in a bottle and you can do with it whatever you want to, whenever you want it," said Michael Grätzel. Such a gas can indeed be burned – in a boiler or engine – releasing only water vapor. It can also pass into a fuel cell to generate electricity on demand. And the 12.3% conversion efficiency achieved at EPFL "will soon get even higher," promised Grätzel.

More powerful cells
These high efficiency values are based on a characteristic of perovskite cells: their ability to generate an open circuit voltage greater than 1 V (silicon cells stop at 0.7 V, for comparison).
"A voltage of 1.7 V or more is required for water electrolysis to occur and to obtain exploitable gases," explained Jingshan Luo. To get these numbers, three or more silicon cells are needed, whereas just two perovskite cells are enough. As a result, there is more efficiency with respect to the surface of the light absorbers required. "This is the first time we have been able to get hydrogen through electrolysis with only two cells!" Luo adds.
The profusion of tiny bubbles escaping from the electrodes as soon as the solar cells are exposed to light say it better than words ever could: the combination of sun and water paves a promising and effervescent way for developing the energy of the future.
More information: "Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts," by J. Luo et al. Science, 2014. www.sciencemag.org/lookup/doi/ … 1126/science.1258307
Journal information: Science
Provided by Ecole Polytechnique Federale de Lausanne