Tiny DNA pyramids enter bacteria easily—and deliver a deadly payload
Bacterial infections usually announce themselves with pain and fever but often can be defeated with antibiotics—and then there are those that are sneaky and hard to beat. Now, scientists have built a new weapon against such pathogens in the form of tiny DNA pyramids. Published in the journal ACS Applied Materials & Interfaces, their study found the nanopyramids can flag bacteria and kill more of them than medicine alone.
David Leong, Jianping Xie and colleagues note that some infectious pathogens can lie in wait, undetectable in the human body or in places that antibiotics have a hard time accessing. Engineered nanomedicine offers a new way to deliver drugs directly into bacterial cells, but the carriers developed so far pose problems such as toxicity. So Leong's team decided to use DNA to build a better, safer drug-delivery tool.
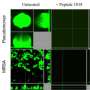
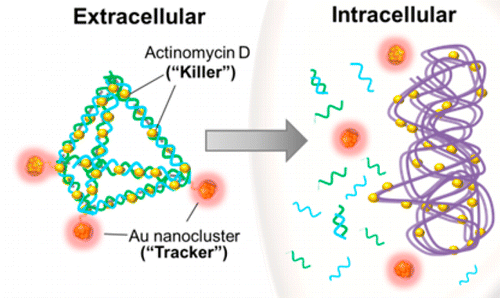
They made little pyramids out of DNA that were so small that thousands could fit in the period at the end of this sentence. Then, they attached gold nanomaterials as fluorescent tags and also packaged the drug actinomycin D (AMD) into the struts of the DNA pyramids. In tests on the common bacteria Escherichia coli and Staphylococcus aureus, the scientists tracked the nanopyramids as they entered the cells and released the drug payload. This killed 65 percent of the S. aureus and 48 percent of the E. coli, compared to 42 percent and 14 percent with AMD alone.
More information: "Novel Theranostic DNA Nanoscaffolds for the Simultaneous Detection and Killing of Escherichia coli and Staphylococcus aureus" ACS Appl. Mater. Interfaces, Article ASAP. DOI: 10.1021/am502591c
Abstract
A novel theranostic platform is made by utilizing a self-assembled DNA nanopyramid (DP) as scaffold for incorporation of both detection and therapeutic moieties to combat bacterial infection. Red-emissive glutathione-protected gold nanoclusters (GSH-Au NCs) were used for bacterial detection. Actinomycin D (AMD) that was intercalated on the DP scaffold was used as therapeutic agent. This results in the formation of theranostic DPAu/AMD. Model bacteria Escherichia coli and Staphylococcus aureus were found to be readily taken in the DPAu/AMD and be susceptible to its killing effect. In addition, DPAu/AMD was observed to outperform the free AMD in killing infectious bacteria. The degradation of the DP structure by DNase was found to be responsible for the release of AMD and the effective killing effect of the infectious bacteria. This novel strategy presents a basic platform for future improvements to detect infectious bacteria and treatment.
Journal information: ACS Applied Materials and Interfaces
Provided by American Chemical Society