Where does the pathological misfolding of the prion originate?
"When they are healthy, they look like tiny spheres; when they are malignant, they appear as cubes" stated Giuseppe Legname, principal investigator of the Prion Biology Laboratory at the Scuola Internazionale Superiore di Studi Avanzati (SISSA) in Trieste, when describing prion proteins. Prions are "misfolded" proteins that cause a group of incurable neurodegenerative diseases, including spongiform encephalopathies (for example, mad cow diseases) and Creutzfeldt-Jakob disease. Legname and coworkers have recently published a detailed analysis of the early mechanisms of misfolding. Their research has just been published in the Journal of the American Chemical Society, the most authoritative scientific journal in the field.
Prions are unique infective agents —unlike viruses, bacteria, fungi and other parasites, prions do not contain either DNA or RNA. Despite their seemingly simple structure, they can propagate their pathological effects like wildfire, by "infecting" normal proteins. PrPSc (the pathological form of the prion protein) can induce normal prion proteins (PrPC) to acquire the wrong conformation and convert into further disease-causing agents.
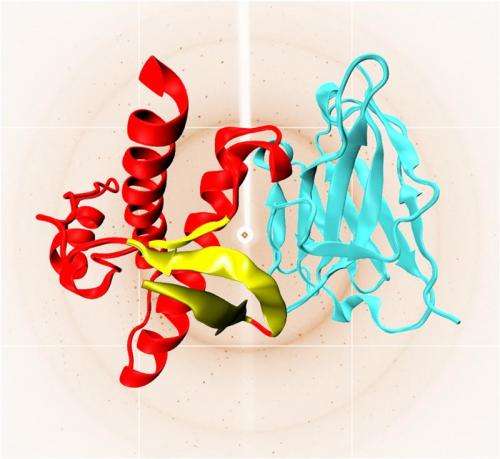
"For the first time, our experimental study has investigated the structural elements leading to the disease-causing conversion" explains Legname. "With the help of X-rays, we observed some synthetic prion proteins engineered in our lab by applying a new approach —we used nanobodies, i.e. small proteins that act as a scaffolding and induce prions to stabilize their structure". Legname and colleagues reported that misfolding originates in a specific part of the protein named "N-terminal". "The prion protein consists of two subunits. The C-terminal has a clearly defined and well-known structure, whereas the unstructured N-terminal is disordered, and still largely unknown. This is the very area where the early prion pathological misfolding occurs" adds Legname. "The looser conformation of the N-terminal likely determines a dynamic structure, which can thus change the protein shape".
"Works like ours are the first, important steps to understand the mechanisms underlying the pathogenic effect of prions" concludes Legname. "Elucidating the misfolding process is essential to the future development of drugs and therapeutic strategies against incurable neurodegenerative diseases".
More information: "Probing the N-Terminal β-Sheet Conversion in the Crystal Structure of the Human Prion Protein Bound to a Nanobody." Romany N. N. Abskharon, Gabriele Giachin, Alexandre Wohlkonig, Sameh H. Soror, Els Pardon, Giuseppe Legname, and Jan Steyaert, Journal of the American Chemical Society 2014 136 (3), 937-944. DOI: 10.1021/ja407527p.
Journal information: Journal of the American Chemical Society
Provided by Sissa Medialab