Using pressure to swell pores, not crush them
More than a decade ago, Thomas Vogt and Yongjae Lee, then colleagues at Brookhaven National Laboratory, uncovered a counter-intuitive property of zeolites. When they put these porous minerals in water, and then put the water under high pressure, the tiny cavities within the zeolites actually grew in size.
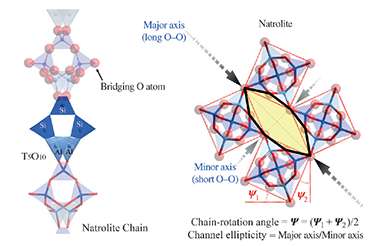
Pressure failed to crush, and even caused expansion. In the years since, Vogt and Lee, now at the University of South Carolina and Yonsei University (Seoul), respectively, have followed up with cation exchange experiments, placing a series of alkali metal ions into the pores of the aluminosilicate zeolites, particularly focusing on natrolite. X-ray diffraction studies, in collaboration with Chi-Chang Kao at Stanford University, have revealed the interior geometry of the cavities and the arrangement of the cations and water molecules held within, before and after pressurization.
The team has just published a detailed characterization of Li+, Na+, K+, Rb+ and Cs+ natrolites, the first four of which, when treated under pressure in water, become "super-hydrated" with water molecules – that is, the process inserts more water molecules into the zeolites than are present under ambient conditions.
The water molecules and ions together adjust the surrounding aluminosilicate framework. The team likens the shift in structure under pressure to what you see when you shift a "chatterbox," the children's fortune teller constructed from paper. The pressure-induced hydration can cause dramatic unit cell volume increases: more than a 20 percent expansion in Li-natrolite, for example.
The phenomenon is more than just an academic curiosity. The team is pursuing a number of applications in which a "tuned" cavity size that is triggered by pressure could be useful. Selectively – and irreversibly – trapping radioactive cations in a nuclear waste stream, for example, is just one area in which they've already demonstrated progress.
More information: dx.doi.org/10.1002/chem.201300591
Provided by University of South Carolina











.jpg)