The path a proton takes: Scenic route preferred to more direct path in alternative fuel cell membrane

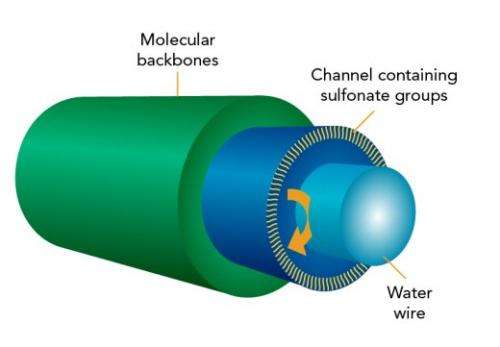
(Phys.org)—Meandering along the water-filled highways laced through an alternative membrane for fuel cells, protons visit sulfur and oxygen atom clusters along the way, according to scientists at Pacific Northwest National Laboratory. The highways are lined with identical hook-shaped molecules. Each channel contains a shell that keeps the water in, formed by the molecules' backbones. The small clusters, known as sulfonate groups, dangle inside the channel. In the middle is a thin "wire" of water.
"Conventional wisdom was that the protons would zip along the center of the water channel, where there were no sulfonate groups to slow them down," said Dr. Ram Devanathan, a materials scientist at PNNL who led the study. "What we found was that the proton doesn't go through the highway, but rather goes on sightseeing trips mainly along the sulfonate groups."
Understanding how protons are transported through membranes could answer one of the key problems of adopting fuel cells. With the potential to revolutionize transportation and portable power, as well as bring electricity to remote locations, fuel cells are a promising energy conversion devices. But, the cells are costly. If a membrane could be designed that withstands higher temperatures and effectively allows protons to pass, fuel cell designers could remove the platinum. Replacing the platinum with a less costly option would drive down the costs.
"Whether you are using membranes for separation or water purification, you are interested in selective transport through the membrane," said Dr. Michel Dupuis, a theoretical chemist and American Association for the Advancement of Science Fellow who worked on the study. "Specifically, we are interested in how changing the design would affect proton transport through the membrane."
The team began with computer simulations of thousands of molecules of the short side chain perfluorosulfonic acid membrane. They wanted to determine what would happen if they changed different aspects of the molecules used to construct the membrane. The team ran simulations where they changed different aspects of the molecule, including the length of the side chain that dangles the sulfonate group into the water wire.
Conventional wisdom said that making the side chains shorter would make the central water channel larger and give the protons more room to move, allowing them to go faster. Surprisingly, the team found that the chain length did not matter. When the chain is long it folds back and doesn't block the channel. When the chain is short, it just stays out of the way.
The simulations were run on supercomputers at EMSL and National Energy Research Scientific Computing Center. "In the experiments, there are a lot of other compounding factors," said Dupuis. "The neat thing about simulations is that you can isolate the factors and just change one, such as the side chain length, while keeping everything else the same."
The results compared well with experimental data collected on the perfluorosulfonic acid membrane.
Right now, Devanathan and Dupuis are conducting detailed studies to determine what influences protons bouncing between the sulfonate groups. They are also examining how to transfer the protons effectively in the absence of water, perhaps with an ionic liquid.
More information: R Devanathan and M Dupuis. 2012. "Insight from Molecular Modelling: Does the Polymer Side Chain Length Matter for Transport Properties of Perfluorosulfonic Acid Membranes?" Physical Chemistry Chemical Physics 14:11281-11295. DOI:10.1039/C2CP24132C.
Journal information: Physical Chemistry Chemical Physics
Provided by Pacific Northwest National Laboratory