Research team gives drug dropouts a second chance
(Phys.org) -- A cross-disciplinary team of researchers at the University of Maryland has designed a molecular container that can hold drug molecules and increase their solubility, in one case up to nearly 3000 times. Their discovery opens the possibility of rehabilitating drug candidates that were insufficiently soluble. It also offers an opportunity to improve successful drugs that could be made even better with better solubility.
The team's innovative findings were recently published in a study in Nature Chemistry, in which the authors note that "the solubility characteristics of 40-70 percent of new drug candidates are so poor that they cannot be formulated on their own, so new methods for increasing drug solubility are highly prized."
![cucurbit[n]urils - or CB[n] n=5,6,7,8,10. UMD team gives drug dropouts a second chance](https://scx1.b-cdn.net/csz/news/800a/2012/umdteamgives.png)
The Maryland scientists where able to increase the solubility of ten insoluble drugs by between 23 and 2,750 times, by forming container-drug complexes. They also show that their containers have low toxicity in human cell line and mice studies, and that the molecular containers can be built from inexpensive and readily available reagents.
"We already are working with drug companies to help them solubilize their interesting drug candidates and hope to get them interested in licensing our technology," says co-leader Volker Briken, an associate professor in the department of cell biology and molecular genetics and also a scientist in the Maryland Pathogen Research Institute.
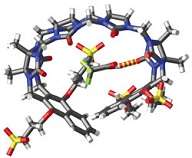
The team, led by Briken and UMD Chemistry & Biochemistry Professor Lyle Isaacs, created their "new class of general-purpose solubilizing agents" based on a type of compound called cucurbit[n]urils - or CB[n]. These are 'macrocyclic' molecules made up of units of bicyclic glycoluril C4H4N4O2 monomers. The n in CB[n] refers to the number of repeat units in the macrocycle.
Many previous attempts have been made to capture drug molecules within these and other synthetic cages and capsules to increase drugs' solubility, but with limited success.
Issacs and Briken say that next their team would like to increase the variety of novel acyclic CBs in order to be able to solubilize a maximal number of small chemical drug candidates, and also would like to generate CBs that can be specifically targeted -- for example to cancer cells.
Provided by University of Maryland