'Killer stainless steel': New process
(Phys.org) -- Stainless steel is the icon of cleanliness for home and commercial kitchens, restaurants, hospitals and other settings, but it can collect disease-causing bacteria like other surfaces if not cleaned often. Scientists now are reporting discovery, in the ACS journal Langmuir, of a practical way to make stainless steel that disinfects itself.
Christophe Detrembleur and colleagues explain that while stainless steel is prized for its durability, resistance to corrosion and ease of cleaning, it readily collects bacteria over time. The bacteria can form invisible colonies or biofilms – collections of colonies bound tightly to a surface – that spread disease. Existing ways of making stainless steel with an antibacterial surface are complicated, expensive and require the use of potentially toxic chemical substances. The authors sought an easier, “greener” way to make an antibacterial coating for stainless steel.
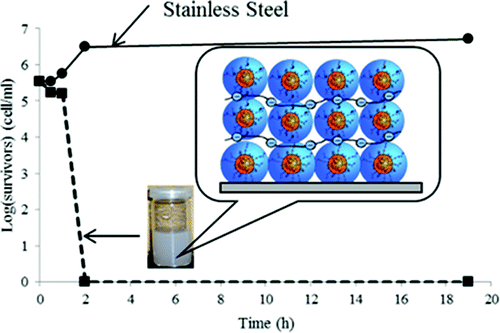
They describe development of a process for giving stainless steel a coating that killed all E. coli bacteria present within two hours in laboratory tests. It involves applying a layer of a bio-inspired adhesive to the steel, then four alternating layers of a negatively-charged polymer and positively-charged polymer micelles containing silver-based particles, which are highly bactericidal. The process takes only 10 minutes and uses water instead of potentially toxic substances. “This novel water-based approach is convenient, simple and attractive for industrial applications,” the researchers say.
More information: Antibacterial Polyelectrolyte Micelles for Coating Stainless Steel, Langmuir, 2012, 28 (18), pp 7233–7241. DOI: 10.1021/la3003965
Abstract
In this study, we report on the original synthesis and characterization of novel antimicrobial coatings for stainless steel by alternating the deposition of aqueous solutions of positively charged polyelectrolyte micelles doped with silver-based nanoparticles with a polyanion. The micelles are formed by electrostatic interaction between two oppositely charged polymers: a polycation bearing 3,4-dihydroxyphenylalanine units (DOPA, a major component of natural adhesives) and a polyanion (poly(styrene sulfonate), PSS) without using any block copolymer. DOPA units are exploited for their well-known ability to anchor to stainless steel and to form and stabilize biocidal silver nanoparticles (Ag0). The chlorine counteranion of the polycation forms and stabilizes biocidal silver chloride nanoparticles (AgCl). We demonstrate that two layers of micelles (alternated by PSS) doped with silver particles are enough to impart to the surface strong antibacterial activity against gram-negative E. coli. Moreover, micelles that are reservoirs of biocidal Ag+ can be easily reactivated after depletion. This novel water-based approach is convenient, simple, and attractive for industrial applications.
Journal information: Langmuir
Provided by American Chemical Society