Planned coincidence: Antibody-based search for new chemical reactions
(Phys.org) -- Many discoveries are made by chance, but it is also possible to help it along: The chance of finding something interesting increases when the number of experiments rises. French researchers have now applied this principle to the search for new chemical reactions. In the journal Angewandte Chemie, they have introduced a new concept based on antibodies and a "sandwich" immunoassay.
Is there any value in randomly mixing substances together like an alchemist to see what happens? When it is carried out systematically and on a large scale, this promising approach, known as high-throughput screening, has become an established technique used in the search for pharmaceutical agents and catalysts. This concept is now being applied more broadly to the search for novel types of chemical reactions, particularly in the search for new, easier, faster, or more elegant synthetic pathways for natural products, specialty chemicals, and drugs.
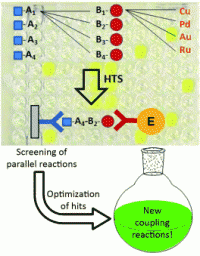
French scientists led by Frédéric Taran (Institute of Biology and Technology, Saclay, iBiTec-S, Gif-sur-Yvette) have now developed a new immunoassay-based approach to searching for new coupling reactions that link two organic molecules together.
Reactants A and B are added to the wells of a microtiter plate. In some wells, various transition metals are added as possible reaction promotors. Reactant A carries a marker that is recognized and bound by antibody AK1; reactant B carries a marker for antibody AK2. If a coupling occurs, the product has both markers. After the reaction, the solutions are transferred to new plates that are coated with AK1. After a washing step, only molecules with a binding site for AK1 remain on the plate. A solution of AK2 is next applied, followed by another washing step. Wherever AK2 binds, a product must be present that carries both markers – the result is a “sandwich” in which the product is the filling between two antibody “slices” of bread. Successful reactions are made visible by an enzyme that is bound to AK2 and causes the color to change to yellow. Wherever the color is clearly yellow, the reaction product is analyzed to determine if the reaction that formed it is of a known type or is previously unknown.
In order to prove that this concept works, the researchers examined 2260 reactions in parallel. The reactants they selected have both conventional and unconventional reactive groups. They were thus able to identify two new types of reaction promoted by copper: the reaction of thioureas to form isoureas and a cyclization reaction to form thiazole derivatives from alkynes and N-hydroxy thioureas.
More information: Frédéric Taran, Reaction Discovery by Using a Sandwich Immunoassay, Angewandte Chemie International Edition, dx.doi.org/10.1002/anie.201201451
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Wiley