Development of a new method for the boron-doping of two dimensional carbon materials
Kyoto University researchers have developed a new method for the boron-doping of two dimensional carbon materials, which is expected to be a promising approach towards the development of highly efficient electron transporting materials for organic electronics.
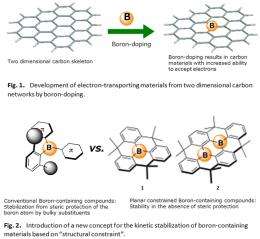
A crucial issue in the field of organic electronics is the development of efficient electron transporting materials. The recent development of hole-transporting materials in the field of in organic photovoltaics has resulted in an improvement of the light-to-electricity conversion efficiency to 10%, even though the electron-transporting materials have been limited almost to fullerene derivatives. The development of new electron-transporting materials is therefore a key step for the development of organic photovoltaic materials with significantly increased light-to-electricity conversion efficiencies. A promising molecular design approach for novel electron-transporting materials is the incorporation of boron atoms (boron-doping) into two dimensional carbon networks (Fig.1). However, in order to successfully implement the concept of "boron-doping" into the development of these materials, the crucial problem of stabilizing the resulting boron-containing organic compounds has to be overcome.
The research group proposed a new concept for the kinetic stabilization of boron-containing materials based on "structural constraint" (Fig.2). They have developed an effective synthetic method for the synthesis of model compounds and showed that a series of corresponding boron-containing carbon materials revealed high electron accepting abilities as well as high stability towards air and heat. These results demonstrate a new paradigm for the kinetic stabilization of boron-containing two dimensional carbon polycyclic skeletons in the absence of bulky aryl groups. These results should furthermore allow the development of a new class of fascinating 2D carbon materials with boron as the key element. The application of this method to boron-embedded graphene, low molecular weight polycyclic carbon materials, as well as fullerenes and carbon nanotubes would lead to the development of excellent electron-transporting materials that can realize higher light-to-electricity conversion efficiencies in organic photovoltaics.
More information:
"Planarized Triarylboranes: Stabilization by Structural Constraint and Their Plane-to-Bowl Conversion"
Zhiguo Zhou, et al. Planarized Triarylboranes: Stabilization by Structural Constraint and Their Plane-to-Bowl Conversion. J. Am. Chem. Soc., Article ASAP Publication Date (Web): February 28, 2012 DOI:10.1021/ja211944q
Provided by Kyoto University