First chemical complex consisting of rare earth metals and boron atoms produces unexpected results

Boron is an intriguing member of the periodic table because it readily forms stable compounds using only six electrons—two fewer than most other main-group elements. This means that chemists can easily add boron to unsaturated hydrocarbons, and then use electron-rich atoms, such as oxygen, to change organoborons into versatile units such as alcohols and esters. Recently, researchers found that combining transition metals with boron ligands produces catalysts powerful enough to transform even fully saturated hydrocarbons into new organic functionalities with high selectivity.
Now, Zhaomin Hou and colleagues from the RIKEN Advanced Science Institute in Wako have made another breakthrough in this field: they have created the first-ever complexes between boron ligands and rare earth metals1. Because these novel chemical combinations display a surprising ability to incorporate molecules such as carbon monoxide into their frameworks, they have potential applications that range from synthesizing organic substrates to controlling noxious emissions.
Rare earth metals are hot commodities because they are vital for products in high demand such as smartphones and electric cars (Fig. 1). However, full chemical studies of these elements are only in their infancy since they are difficult to handle under normal conditions.
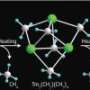
According to Hou, typical methods to prepare transition metal–boron complexes—halogen or metal exchange reactions, for example—seemed unsuitable for rare earth metals. Instead, the team used a vigorous lithium–boron compound to handle the reactive rare earth precursors, producing previously unseen scandium–(Sc–B) and gadolinium–boron (Gd–B) complexes in good yields, but not without difficulty. “Rare earth–boron compounds are air- and moisture-sensitive and sometimes thermally unstable,” says Hou. “They therefore require great care in isolation and handling.”
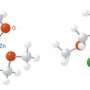
To determine whether or not the Sc–B complex could act as a nucleophile—an important electron-donating reagent in organic chemistry—the team reacted it with N,N,-diisopropylcarbodiimide, a molecule that easily accepts electrons to change into an amidinate salt. X-ray analysis revealed that initially, the carbodiimide became incorporated between Sc and carbon ligands on the rare earth metal, but extra quantities of the reagent became incorporated between the Sc–B bond. Furthermore, adding carbon monoxide to this mixture also caused a rare earth–boron insertion, accompanied by an unexpected rearrangement into a cyclic structure.
Because chemists rely on insertion reactions to efficiently transform ligands into a diverse range of products, these findings should enable development of brand new synthetic techniques—opportunities that Hou and his team are actively pursuing.
More information: Li, S., et al. Rare earth metal boryl complexes: Synthesis, structure, and insertion chemistry. Angewandte Chemie International Edition 50, 6360–6363 (2011).
Provided by RIKEN