A possible fix for misfolding proteins
(PhysOrg.com) -- Troubled proteins in need of rescue may someday have a champion in a common drug used to treat high blood pressure.
The Rice University laboratory of bioengineer Laura Segatori reported today that research involving lacidipine, a calcium channel blocker also known by brand names Lacipil and Motens, could be a key to helping people who suffer from an incurable, neuropathic form of Gaucher disease. This inherited metabolic disorder is characterized by accumulations of a fatty substance in cells and certain organs that can prevent them from functioning properly.
The paper by Segatori, Rice's T.N. Law Assistant Professor in Chemical and Biomolecular Engineering, and graduate student Fan Wang and Rice senior Ann Chou appears in today's online edition of Chemistry and Biology.
Segatori's research focuses on the misfolding of proteins, workhorses in the body that determine what cells and organs do and how they do it. Proteins start as chains of amino acids that snap in an instant into distinct configurations, a process that remains one of biology's great mysteries but one that Segatori and her peers are figuring out, bit by bit.
Proteins often misfold even in the healthiest persons, Segatori said, and cells have an elegant, efficient system for eliminating misfolded proteins and other refuse. But the system can break down.
In Gaucher disease, proteins containing destabilizing mutations misfold and are degraded very quickly. Loss of these proteins, which normally traffic to the lysosome and catalyze the degradation of lipids, results in buildup of these lipids; this can lead to such problems as a malfunctioning liver, enlarged spleen, skeletal disorders, anemia and neurological disorders.
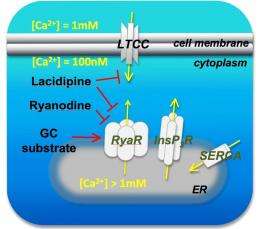
The Rice researchers worked with fibroblasts taken from skin lesions of people with Gaucher. They found that lacidipine enhances the protein-folding mechanism by modulating calcium levels and regulating the movement of signaling calcium ions.
Segatori said impairment of calcium homeostasis further compromises the folding of already destabilized, mutated versions of the enzyme glucocerebrosidase (GC). Slowing the folding process ever so slightly by regulating calcium stabilizes GC and lets it fold properly and enter the lysosome, where it breaks down lipids.
"If you can force the folding to occur, you can rescue native folding of mutant proteins, which has been shown to lead to restored activity," Segatori said.
Segatori and Wang hope their work opens the door to possible treatments for neuropathic diseases that will be easier on patients and less expensive than enzyme replacement therapy, which involves injecting recombinant protein. Segatori said lacidipine has three distinct advantages: It is nontoxic to cells, is a small molecule that readily crosses the blood/brain barrier and is approved for use in humans by the Food and Drug Administration.
On the other hand, the researchers have not yet studied the effect of lacidipine on neurons. "We don't want to say we can cure this disease with calcium blockers, but they are a good tool for research," Segatori said. "Essentially, we treat cells with this molecule and see if we rescued the protein activity. If we did, we can then work to understand what the molecule actually did to the folding machinery of the cell."
She also cautioned that calcium blockers might have side effects. "Their response in the cell is quite broad. That's why I'm hesitant to say that this could be a cure for Gaucher disease. Maybe we're rescuing the folding of that enzyme, but we don't know what else we might be doing."
But the positive implications go beyond Gaucher. "There is possibly an avenue to use calcium blockers to further the study and treatment of other types of misfolding diseases," Segatori said. "Similar studies have been conducted using calcium blockers in neurons of Parkinson's patients. The results are highly promising. And there's also a lot of interest in the correlation between Parkinson's and Gaucher diseases, because it seems like a lot of people who have Gaucher are at risk for Parkinson's disease."
Gaucher disease occurs in about 1 in 50,000 to 1 in 100,000 people. Treatment for the more common types of the disease involves enzyme replacement therapy.
More information: Lacidipine Remodels Protein Folding and Ca2+ Homeostasis in Gaucher's Disease Fibroblasts: A Mechanism to Rescue Mutant Glucocerebrosidase, Chemistry & Biology, Volume 18, Issue 6, 766-776, 24 June 2011 DOI:10.1016/j.chembiol.2011.04.008
Abstract
The hallmark of Gaucher's disease cellular pathogenesis is the lysosomal accumulation of glucosylceramide, which is caused by misfolding of mutated glucocerebrosidase (GC) and loss of lysosomal GC activity, and leads to depletion of [Ca2+]ER. We demonstrate that modulation of Ca2+ homeostasis and enhancement of the cellular folding capacity synergize to rescue the folding of mutated GC variants. Lacidipine, an L-type Ca2+ channel blocker that also inhibits [Ca2+]ER efflux, enhances folding, trafficking, and activity of degradation-prone GC variants. Lacidipine remodels mutated GC proteostasis by simultaneously activating a series of distinct molecular mechanisms, namely modulation of Ca2+ homeostasis, upregulation of the ER chaperone BiP, and moderate induction of the unfolded protein response. However, unlike previously reported proteostasis regulators, lacidipine treatment is not cytotoxic but prevents apoptosis induction typically associated with sustained activation of the unfolded protein response.
Provided by Rice University
















