Air-fueled battery could last up to 10 times longer
A new type of air-fuelled battery could give up to ten times the energy storage of designs currently available.
This step-change in capacity could pave the way for a new generation of electric cars, mobile phones and laptops.
The research work, funded by the Engineering and Physical Sciences Research Council (EPSRC), is being led by researchers at the University of St Andrews with partners at Strathclyde and Newcastle.
The new design has the potential to improve the performance of portable electronic products and give a major boost to the renewable energy industry. The batteries will enable a constant electrical output from sources such as wind or solar, which stop generating when the weather changes or night falls.
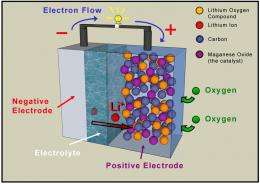
Improved capacity is thanks to the addition of a component that uses oxygen drawn from the air during discharge, replacing one chemical constituent used in rechargeable batteries today. Not having to carry the chemicals around in the battery offers more energy for the same size battery. Reducing the size and weight of batteries with the necessary charge capacity has been a long-running battle for developers of electric cars.
The STAIR (St Andrews Air) cell should be cheaper than today's rechargeables too. The new component is made of porous carbon, which is far less expensive than the lithium cobalt oxide it replaces.
This four-year research project, which reaches its halfway mark in July, builds on the discovery at the university that the carbon component's interaction with air can be repeated, creating a cycle of charge and discharge. Subsequent work has more than tripled the capacity to store charge in the STAIR cell.
Principal investigator on the project, Professor Peter Bruce of the Chemistry Department at the University of St Andrews, says: "Our target is to get a five to ten fold increase in storage capacity, which is beyond the horizon of current lithium batteries. Our results so far are very encouraging and have far exceeded our expectations."
"The key is to use oxygen in the air as a re-agent, rather than carry the necessary chemicals around inside the battery," says Bruce.
The oxygen, which will be drawn in through a surface of the battery exposed to air, reacts within the pores of the carbon to discharge the battery. "Not only is this part of the process free, the carbon component is much cheaper than current technology," says Bruce. He estimates that it will be at least five years before the STAIR cell is commercially available.
The project is focused on understanding more about how the chemical reaction of the battery works and investigating how to improve it. The research team is also working towards making a STAIR cell prototype suited, in the first instance, for small applications, such as mobile phones or MP3 players.
Source: Engineering and Physical Sciences Research Council (news : web)