Hydrogen bonds in the polymer phase boost important properties of nacre mimetics
Nacre, or mother of pearl, has highly attractive mechanical properties but cannot be processed into larger-scale structures. Synthetic nanocomposites can mimic the characteristic brick-and-mortar-like structure of nacre, but combinations of stiffness, strength, toughness and desirable optical properties have remained difficult to achieve. Scientists based in Aachen, Germany, report in the journal Angewandte Chemie that the introduction of tailored hydrogen bonds in the polymer mortar by macromolecular engineering leads to an unprecedented combination of the relevant properties, which are perfectly tunable.
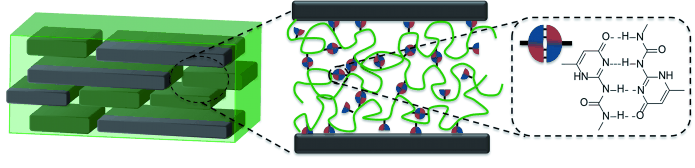
In nature, nacre is a nanocomposite made of layers of inorganic microtablets laminated by different biopolymers that stabilize the architecture. Mankind has always used nacre for decorative purposes, but could not exploit it industrially, despite its generally favorable mechanical properties. Andreas Walther and his team at the DWI - Leibniz Institute for Interactive Materials, Germany, in collaboration with KIT in Karlsruhe, Germany, use a macromolecular engineering approach to mimic and tweak the nacre nanocomposite structure for possible mechanical and functional applications. Focusing on the laminating polymer phase, they designed a low-molecular-weight polymer with low glass-transition temperature, which was equipped with varying degrees of a supramolecular binding motif. Combined with advanced synthetic nanoclay platelets, the nanocomposite material self-assembled to form a film that possesses all relevant features like excellent transparency, structural periodicity, orientation, stiffness, and a favorable fracture behavior including self-healing ability.
Key to the success are the supramolecular bonds within the soft polymer matrix. The scientists chose a ureidopyrimidinone (Upy) entity as the bonding motif that, like the nucleobases in DNA, forms dimers through hydrogen bonds. "The type and amount of hydrogen bonds allow us to tune the manner how the transition of elastic to plastic deformation occurs", Walther explains. "In contrast to covalent bonds, supramolecular bonds can first provide resistance against deformation (stiffness boost), but at substantial stress levels, the bonds can open up and provide fracture energy dissipation by stick/slip interactions and frictional sliding of the platelets against each other."
These so-called sacrificial bonds allow full control over the material on different levels, because, depending on their amount, the material can transform from high stiffness and strength to desired combinations of high stiffness and toughness, as the authors write. So, upon stress the material with 13% Upy motif displayed toughening and failure phenomena "very reminiscent of the behavior of highly reinforced biological materials." The scientists further showed that the films are excellent gas barriers. This opens up further possibilities, as Walther says: "The materials are not only appealing as mechanically robust nanocomposites, but also for their multifunctional properties relevant to other applications: as fully transparent oxygen barrier films to encapsulate organic electronics, or to protect against fire with halogen- and heavy-metal-free compositions."
More information: "Hierarchical Nacre Mimetics with Synergistic Mechanical Properties by Control of Molecular Interactions in Self-Healing Polymers." Angew. Chem. Int. Ed.. doi: 10.1002/anie.201502323
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Wiley





















