A turning point in the physics of blood
Mike Graham knows that fluid dynamics can reveal much about how the flow of blood helps and hinders individual blood cells as they go about their work.
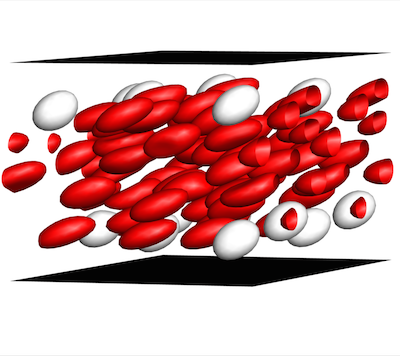
Graham, the Vilas Distinguished Achievement Professor and Harvey D. Spangler Professor of Chemical and Biological Engineering at UW-Madison, established a theoretical basis for these ideas by creating complex computer stimulations that show how relatively stiff white blood cells and platelets interact with more flexible red blood cells. As the different cells collide during blood flow, white cells tend to be pushed toward the walls of a blood vessel. This segregation process, called margination, creates some advantages—for example, letting white blood cells quickly exit the blood vessel to head to the site of an injury or infection.
However, the mechanical details of blood could spell both good news and bad in areas ranging from drug delivery to blood disorders to the spread of disease. "I view my role as providing a fundamental basis of understanding for practitioners and for other engineers who are more directly connected with applications," Graham says.
Graham's work has reached a crucial turning point, yielding a theory that opens a door for other researchers to take advantage of what Graham's group has learned and observed throughout years of computationally simulating the physics of blood flow. In a paper published May 4, 2015, in the journal Physical Review Letters, Graham and PhD students Kushal Sinha and Rafael G. Henríquez Rivera lay out an equation that yields simple predictions as to how quickly blood cells will migrate away from blood-vessel walls, how they will behave when they collide with each other and accordingly how they will segregate during flow.
In the long run, these insights could help practitioners manipulate the mechanics of blood to design better blood-transfusion treatments, craft techniques for delivering drugs through small particles in the blood, and perhaps even devise processes for isolating blood-borne tumor cells that spread cancer throughout the body.
"I'm really excited about this paper, because it's the first analytical theory for this phenomenon," Graham says. "It's not very common that theory is ahead of experiments, but we're in that position now."
That said, Graham is proceeding on both fronts to draw a firmer connection between these mechanical insights and the many biological functions they might impact. Moving ahead, his group will both refine the new equation to suit more complex flow situations and pursue an experimental collaboration with Wilbur Lam, a hematologist at Georgia Tech and Emory University in Atlanta.
Building on Graham's theoretical and simulation work, Lam's research group is creating microfluidic devices to study the behavior of blood cells in essentially their natural habitat. Lam has developed a way to grow endothelial cells—which line the interior walls of our blood vessels—inside the artificial channels of the microfluidic devices. "I think together our labs have really stumbled on how fluid mechanics may be able to explain a lot of the biological phenomena we see in blood," Lam says. "This can be related to a new way of understanding inflammation, infections, even transfusion medicine. It really pervades many different problems we see in hematology."
Even in developing the computational and theoretical side of the work, Graham thinks a great deal about making sure his work is credible to medical practitioners. That is especially challenging for a researcher who deals in computer simulations, because a simulation simply can't factor in every single variable without requiring a prohibitive amount of computing resources.
However, Graham says that capturing the physical nuances of blood vessels' shape, size and relative stiffness has tremendous value, even given the myriad other forces at work in the human body.
"We'd like to be able to convince practitioners that you don't have to worry about all the details to capture the fundamental understanding of what's going on," Graham says. "It's extremely challenging to incorporate all the phenomena that might be important into a simulation. You have to make your case convincingly, if you want somebody to apply this research, that you've kept the important parts."
Both researchers point out that sickle cell anemia has long been understood as both a mechanical and a biological problem. The defective red blood cells the disease causes are not only misshapen, but also stiffer than healthy red blood cells, meaning they block blood flow. Yet on a more detailed mechanical level, Graham and Lam say, it may even be that sickle cells literally poke and irritate the inner walls of blood vessels. If so, that would make sickle cell anemia not just a blood disorder but a disorder of the entire circulatory system. Their combined research strengths now create an opportunity to test that hypothesis. "Biologists and hematologists have known for decades that these cells can get stuck, but what is less understood is that the blood vessel walls in the entire patient are really inflamed, and we don't really know why," Lam says.
The researchers say a better understanding of blood-flow mechanics also could help to make blood transfusions safer. Transfusions can sometimes set off heart attacks or lung damage, and the medical community isn't entirely sure why. Lam wants to find out if certain cells in stored donated blood have mechanical properties that put patients at greater risk.
Though his collaboration with Lam is still in an early stage, both researchers see the possibility of opening a new frontier in blood research.
"This would be a whole new category of things we could be looking at, and that's why it's so exciting," Lam says. "Suddenly we have applications where the mechanics can be just as important."
Journal information: Physical Review Letters
Provided by University of Wisconsin-Madison