Refined CRISPR/Cas genome editing accelerates generation of transgenic mice
Although the genome editing system known as CRISPR/Cas has revolutionized genetic research in cell lines, its overall efficiency has been relatively poor when used to generate genetically altered animals for disease modeling. Now Whitehead Institute scientists have altered the approach in a manner that could accelerate the production of mice carrying precise mutations of multiple genes.
The clustered, regularly interspaced, short palindromic repeats (CRISPR)-associated protein 9 (Cas) system precisely edits a genome by creating breaks in the DNA at specific locations and then relying on the cell's DNA repair mechanisms to insert or remove one or more targeted genes. The CRISPR/Cas system has made genome editing in cell lines relatively quick and inexpensive.
As previously shown by Whitehead Founding Member Rudolf Jaenisch's lab, the system can be used for genetic editing in animals—albeit with some difficulty. In such applications, CRISPR/Cas-mediated HDR occurs far below 20%, thus hampering the production of transgenic animals (typically mice) in numbers sufficient for modeling human diseases.
In this week's issue of Nature Biotechnology, postdoctoral researcher Takeshi Maruyama and other researchers in the lab of Whitehead Member Hidde Ploegh present a refinement of the CRISPR/Cas system.
"This is an immature field, and it is developing so quickly," says Maruyama. "We needed some optimization to make it more practical for researchers who use mice in their research. I think this is a big improvement to how we make transgenic mice."
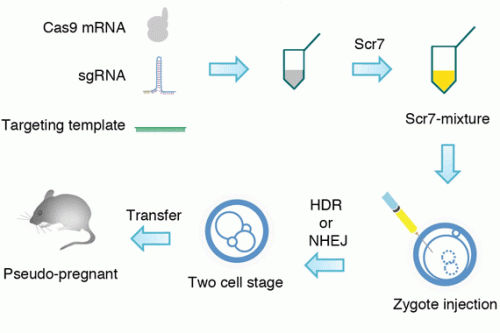
Maruyama notes that almost all cells and organisms use two mechanisms of DNA repair—non-homologous end joining (NHEJ) and homology-directed repair (HDR). When left to their own devices, cells rely on NHEJ more often than HDR, which is the more accurate mechanism preferred when using CRISPR/Cas.
Intent on increasing HDR's activity, Maruyama added the anticancer drug Scr7 to the cells. Scr7 binds to Ligase IV, a key enzyme in the main NHEJ pathway, and stalls this type of DNA repair. The more accurate HDR mechanism picks up the slack, increasing the CRISPR/Cas' efficiency in the process. The tweak works not only in cell lines, but also in fertilized zygotes used to create transgenic mice.
"This will really improve how we generate mice with the specific mutations that we'd like to study", says Maruyama, who will be starting soon as an assistant professor at Hokkaido University. "Before, getting the mice we needed for an experiment was very time-consuming. This will speed up our research. In addition, the drug-based approach might enable us to generate mutant mice carrying more complicated mutations."
More information: "Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining." Nature Biotechnology (2015) DOI: 10.1038/nbt.3190
Journal information: Nature Biotechnology
Provided by Whitehead Institute for Biomedical Research