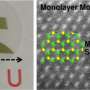
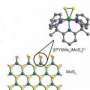
Moving toward a cheaper, better catalyst for hydrogen production

(Phys.org) —Hydrogen could be an important source of clean energy, and the cleanest way to produce hydrogen gas is to split water into hydrogen and oxygen. But the catalyst currently used to facilitate this water-splitting reaction is platinum. And that's a problem.
When an electric current is run through water, it can split some of the water molecules. A catalyst lowers the amount of energy needed to split those molecules, and platinum is really, really good at this.
But platinum is also really, really expensive – much too expensive for widespread use in hydrogen production.
So, researchers have long viewed molybdenum sulfide (MoS2) as a promising, much cheaper alternative to platinum. The drawback is that MoS2's catalytic performance is far worse than platinum's. To get around that problem, researchers have been trying to find ways to improve MoS2's catalytic performance. And now they may be on to something.
"The biggest stumbling block to improving MoS2's performance has been a lack of understanding of the connection between the material's performance and its composition and structure," says Linyou Cao, senior author of a new paper on the subject and a materials science and engineering researcher at NC State. "We're now able to shed some light on that connection."
In molybdenum sulfide, the ratio of sulfur atoms to molybdenum atoms can range from two to three. As a result, many researchers wondered if the precise composition of the material could affect its catalytic performance.
According to a new paper from Cao and his team, it doesn't. But the crystalline structure of the material does.
There are three qualities to bear in mind when thinking about catalytic performance:
- Tafel slope: this is how fast the hydrogen evolution rate could increase with the electrical potential. (The lower the Tafel slope, the fewer volts you need to get a targeted hydrogen production rate.)
- Exchange current density: this is the intrinsic catalytic activity a material has when no electrical potential is present (i.e., how much it encourages a hydrogen atom to split off a water molecule in the absence of a current).
- Stability: this is how long the catalyst can perform before it degrades and stops working. (If it burns out immediately, it's not very useful.)
Cao's team discovered that MoS2 with low quality, or amorphous, structures have lower Tafel slopes (which is good), but also have lower exchange current density and stability (which are bad). Conversely, MoS2 with high quality, or single crystal, structures have higher Tafel slopes (which is bad), but much better exchange current density and stability.
"Now that we understand this, we need to carefully engineer MoS2 with a balanced structure to control the effects on all of the aspects of catalysis," Cao says.
The recent work also shows that a collection of MoS2 nanoclusters, which are 5 to30 nanometers in diameter, is the most promising structure for improved catalytic performance. (To picture a collection of nanoclusters, think of the rings of Saturn – a collection of objects in such close proximity to each other that they resemble a solid, until you look very closely.)
The work is discussed in a paper, "Engineering the Composition and Crystallinity of Molybdenum Sulfide for High-performance Electrocatalytic Hydrogen Evolution," published online Dec. 4 in ACS Catalysis.
Publication details
"Engineering the Composition and Crystallinity of Molybdenum Sulfide for High-performance Electrocatalytic Hydrogen Evolution." ACS Catal., Just Accepted Manuscript DOI: 10.1021/cs501635v
Journal information: ACS Catalysis
Provided by North Carolina State University