A first-of-its-kind discovery with an X-ray laser
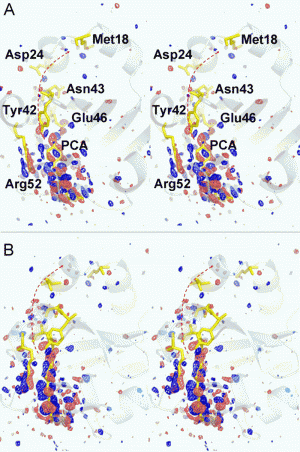
A research team led by physicists at the University of Wisconsin-Milwaukee (UWM) has proven a method that makes it possible to find the atomic structure of proteins in action by producing "snapshots" of them with unprecedented spatial and temporal resolution.
What made it possible were the ultra-short X-ray pulses of a Free Electron Laser (XFEL).
Physics professor Marius Schmidt and doctoral student Jason Tenboer recently completed the experiment with the XFEL at the Stanford Linear Accelerator Center (SLAC) in California.
It confirms that the XFEL imaging method, called time-resolved serial femtosecond crystallography (TR-SFX), can unmask protein structures that have never been seen before, determine what each protein does and reveal how they work together to carry out nearly every function in a living organism.
The experiment also involved researchers from Arizona State University, SUNY Buffalo, University of Chicago, Imperial College London, Lawrence Livermore National Laboratory, Stanford Linear Accelerator Center, and the Deutsches Elektronen-Synchrotron (DESY). Results are published today in the journal Science.
Structure equals function
The successful test of the method they used has opened the door to discovering what Schmidt calls "some of the grand challenges of biology." Proteins are behind almost everything that happens in a living organism, and they play a pivotal role not only in human health, but also in issues as diverse as food, drug discovery and energy.
"We want to understand the molecular basis of life," he says.
But advances are only possible if scientists know what each protein does. For that, they have to know the structure - the arrangements of the atoms in each and how they change when proteins work together.
"We could observe reactions in certain proteins before," says Schmidt, "but our new results show that we can now investigate reactions in almost all proteins."
X-ray crystallography is the method of choice to image proteins with near atomic resolution: X-rays are shot at a protein crystal and diffract off in many directions, creating a pattern of dots the way a single shake of a paintbrush will spray splotches of paint on a wall. The pattern is a kind of fingerprint for that protein.
The millions of data points can be mathematically reconstructed to form a three-dimensional image of the atomic structure at a single point in time.
Using the XFEL as the light source, this kind of imaging is improved from previous equipment.
Change in an instant
Schmidt and Tenboer employed a "pump and probe" experiment, first inducing a chemical reaction in a protein crystal the size of a bacterium using an optical laser to get the atoms moving. Immediately afterward, the X-rays bombarded the crystal, forming the diffraction patterns.
One experiment is over in less time than it takes to blink, but in that span, protein changes can be documented.
From this data, the researchers obtained high-resolution "maps" of time-resolved differences in the electron density, the cloud of electrons in molecules that shifts around during a reaction.
With the XFEL at their X-ray source, Schmidt and Tenboer have overcome limitations with previous methods to follow these shifts.
The XFEL's ultra-quick X-ray pulses makes it possible to collect imaging data during a very short time span - nearly instantaneous - and record the change that occurred in the structure as proteins perform their function.
"Biology happens at inconceivably short time spans," says Tenboer. "So the XFEL at Stanford allows us to do time-resolved studies of proteins in action down to the femtosecond time scale - that's 10 -15 of a second. The blink of an eye probably happens on a millisecond time scale; so you're still talking about twelve orders of magnitude faster."
Also, because the XFEL is a billion times brighter than any existing equipment, scientists can use much smaller crystals, even those at nanoscale, which are easier to form. Laser light used to start a reaction in these very small crystals can penetrate fully through the entire crystal and uniformly initiate a reaction in them.
Both the incredible pulse speed of the XFEL and the strength of the reaction initiated by the optical laser boosted the signal, revealing finer detail.
"This is essential to show unambiguously the structural changes," Schmidt says.
The next step for the research group is to perform a faster "pump and probe" experiment. With an X-ray pulse of 40 femtoseconds, they hope to see step-by-step changes in the resulting images and evidence of the very first elementary steps that lead to the function of these proteins.
More information: Science 5 December 2014: Vol. 346 no. 6214 pp. 1242-1246. DOI: 10.1126/science.1259357
Journal information: Science
Provided by University of Wisconsin - Milwaukee



















