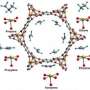
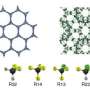
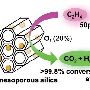Cherry picking molecules based on their Pi electrons
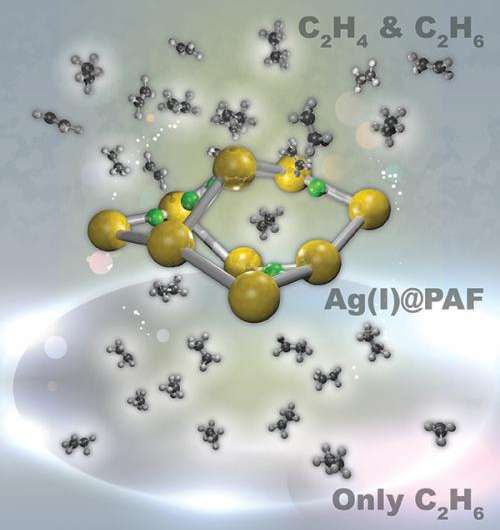
Specialized windshield glass, everyday plastic water bottles, and countless other products are based on ethylene, a simple two-carbon molecule, which requires an energy-intense separation process to pluck the desired chemical away from nearly identical ethane. To eliminate the extreme cooling required in the separation, an international team including researchers at Pacific Northwest National Laboratory designed a material with a porous framework that greatly prefers ethylene. What makes this material particularly potent for applications is that the highly selective sorbent is stable in air and water. In addition, the framework offers a high surface area that speeds the sorting. The material contains silver that binds with the electrons around ethylene's double-bonded carbon atoms. These electrons are known as π electrons or the π cloud.
Every year, billions of tons of ethylene are produced by steam cracking and thermally decomposing ethane. Because ethylene and ethane are roughly the same size and become gases at nearly the same temperature, separating the molecules is extremely difficult on a large scale. Currently, manufacturers distill off the ethylene at high pressures and low temperatures, 23 bar and −25 °C, respectively. This study describes a promising material that does not require extreme pressures or temperatures and far surpasses current zeolites, a popular class of aluminosilicate catalysts, and any other modified framework reported in the scientific literature.
"Compared to existing materials, this is the best material," said Dr. Praveen Thallapally, a materials scientist at PNNL who is part of this work along with Jian Liu, an engineer, and Laboratory Fellow Jun Liu.
The researchers created the silver-based porous aromatic framework by adding π-complexation or the ability to form a bond between an aromatic ring in the framework with the π electrons in the ethylene. They characterized the material using infrared spectroscopy and other instruments at different facilities. After analyzing the data, the team found that the PAF-1-SO3Ag at 296 K or ~22 °C is exceptionally selective. It also has a high surface area, 4000 to 5000 square meters per gram. Additional experiments, supported by engineering simulations at the University of Amsterdam, indicate that PAF-1-SO3Ag could produce batches of 99.95%+ pure ethylene.
The material presents several challenges, however. For example, while the porous aromatic framework (PAF) material easily sheds the ethylene when the conditions are right, at room temperatures the ethylene bonds too tightly to the framework. This tighter bonding makes it difficult to remove and use the ethylene. The material holds promise, although there are challenges in both the manufacturing process and the material's performance, the material paves the way to develop silver-based PAFs that perform complex separations based on pi electrons.
The team is taking on one of the material's challenges: its density. A single gram of the material occupies more space than is practical for larger scale uses. Researchers from the team are working to reduce the material's volume while maintaining its capacity for separating out the desired molecules in tough mixtures.
More information: Li B, Y Zhang, R Krishna, K Yao, Y Han, Z Wu, D Ma, Z Shi, T Pham, B Space, J Liu, PK Thallapally, J Liu, M Chrzanowski, and S Ma. 2014. "Introduction of π-Complexation into Porous Aromatic Framework for Highly Selective Adsorption of Ethylene over Ethane." Journal of the American Chemical Society 136(24):8654-8660. DOI: 10.1021/ja502119z
Journal information: Journal of the American Chemical Society
Provided by Pacific Northwest National Laboratory