Chemists develop novel catalyst with two functions
Chemists at the Ruhr-Universität Bochum have made a decisive step towards more cost-efficient regenerative fuel cells and rechargeable metal-air batteries. They developed a new type of catalyst on the basis of carbon, which can facilitate two opposite reactions: electrolysis of water and combustion of hydrogen with oxygen. A catalyst of this kind might make the storage of wind and solar energy and the manufacture of cost-efficient batteries, for example for electric cars, possible. The team published their report in the "International Edition" of the magazine Angewandte Chemie.
Switching from electrolysis to combustion
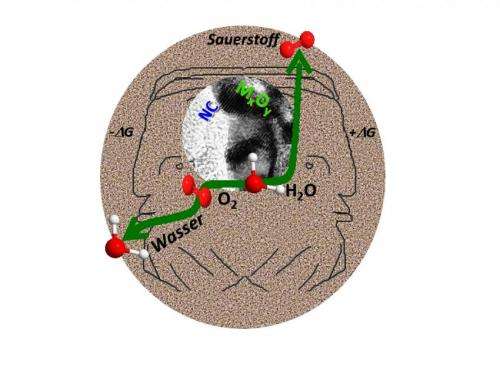
When energy is supplied, the so-called bi-functional catalysts can split water into hydrogen and oxygen– referred to as electrolysis. They can then store the energy in the chemical bonds of the thus formed hydrogen. The same catalysts can also have their polarity reversed to become fuel cells; they combust hydrogen with oxygen to water, generating electricity at the same time. So far, researchers have been using noble-metal catalysts for this purpose. However, these catalysts have the disadvantage of being either good for electrolysis or good for combustion, but not for both.
Carbon-based catalysts
The novel catalysts from Bochum are made from manganese-oxide or cobalt-oxide nano particles which are embedded in specially modified carbon, into which the researchers have integrated nitrogen atoms in specific positions. The team headed by Prof Dr Wolfgang Schuhmann and Prof Dr Martin Muhler from the Faculty of Chemistry and Biochemistry analysed the catalysts using a number of spectroscopic and electrochemical methods. They have thus determined which properties are essential for bi-functionality.
"Irresistibly simple" to manufacture
In a previous publication in the Journal of the American Chemical Society, the researchers from Bochum described another approach for manufacturing bi-functional carbon catalysts. To this end, they had "cut open" carbon nanotubes by applying thermal energy and oxygen, thus rendering the catalyst particles embedded therein usable. "This method is irresistibly simple," says Martin Muhler. Compared to noble-metal catalysts, the production would be highly cost-efficient.
More information: A. Zhao, J. Masa, W. Xia, A. Maljusch, M.-G. Willinger, G. Clavel, K. Xie, R. Schlögl, W. Schuhmann, M. Muhler (2014): Spinel Mn-Co oxide in N-doped carbon nanotubes as a bifunctional electrocatalyst synthesized by oxidative cutting, Journal of the American Chemical Society, DOI: 10.1021/ja502532y
J. Masa, W. Xia, I. Sinev, A. Zhao, Z. Sun, S. Grützke, P. Weide, M. Muhler, W. Schuhmann (2014): MnxOy/NC and CoxOy/NC nanoparticles embedded in a nitrogen-doped carbon matrix for high-performance bifunctional oxygen electrodes, Angewandte Chemie International Edition, DOI: 10.1002/anie.201402710
Journal information: Angewandte Chemie , Journal of the American Chemical Society , Angewandte Chemie International Edition
Provided by Ruhr-Universitaet-Bochum