New tagging technique enhances view of living cells
Scientists hoping to understand how cells work may get a boost from a new technique to tag and image proteins within living mammalian cells.
The new technique, developed by a research team led by University of Illinois at Chicago assistant professor of chemistry Lawrence Miller, provides the clearest, most dynamic view yet of protein-protein interactions in cells when viewed through a specially modified microscope.
The finding is reported in the Proceedings of the National Academy of Sciences (advanced online July 19.)
Knowing where and when particular proteins interact within the cell is key to understanding life processes at the molecular level.
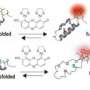
In a technique called luminescence resonance energy transfer, two proteins in a cell are labeled with differently colored, luminescent molecules that absorb light of one color and give it off as another color. By taking several pictures of the cell and mathematically analyzing the pictures, researchers gain information about the proteins' precise location and whether they are interacting.
Miller and his team used a novel type of luminescent molecule for labeling, making it possible to get the same information using fewer pictures. This simplifies the analysis and allows for five-fold faster data acquisition. Images show a 50-fold improvement in sensitivity.
Working with Jerrold Turner, professor and associate head of pathology at the University of Chicago, Miller used a hybrid chemical/genetic approach to tag the proteins of interest. One of the proteins was genetically modified so that it would bind to a terbium complex. The terbium complex has an unusually long time between light absorption and emission. The second target protein was genetically modified to link to a fluorescent tag with a short emission lifetime. When the two proteins interact, the luminescent tags are brought very close together, generating a unique luminescent signal that can be seen under a microscope.
Miller and his colleagues modified a conventional microscope to exploit the long lifetime of the terbium protein tags. Pulsed light is used to trigger the terbium luminescence, detected after the other luminescent species within cells have gone dark, allowing unwanted background to be removed from the image.
The new technique "increases sensitivity and makes the whole process faster," Miller said. "This increases the time-resolution of the experiment, allowing you to see how interactions change on a faster time scale, which can help to better figure out how certain biological phenomena work."
The technique required a reliable way to deliver the luminescent terbium probe through a living cell membrane without contaminating or damaging the cell. The researchers developed a way to co-opt pinocytosis, the process by which cells drink in small amounts of surrounding fluid.
"With this new tool, we hope cell biologists and others will be able to study things they haven't seen before, such as interactions that couldn't be visualized in live cells in real time," Miller said. "Hopefully the method will yield information that makes it easier to deduce biological mechanisms."
Provided by University of Illinois at Chicago