This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Preparation strategies and applications of high-entropy materials catalysts for electrochemical water electrolysis
The escalating consumption of fossil fuels has raised concerns over energy crises and environmental pollution, prompting an urgent need for renewable and eco-friendly energy sources. Hydrogen, with its zero-carbon emissions and high energy density, emerges as an ideal alternative to traditional energy sources.
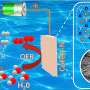
Electrochemical water electrolysis, a key method for green hydrogen production, has been at the forefront of research. However, the high cost and limited availability of precious metal-based catalysts have hindered their widespread application.
The development of high-performance, cost-effective non-noble metal catalysts is an urgent task, leading to the exploration of high-entropy materials (HEMs) as potential game-changers in the field of electrocatalysis.
Researchers (Simiao Sha, Riyue Ge, Ying Li, Julie M. Cairney, Rongkun Zheng, Sean Li, Bin Liu, Jiujun Zhang, Wenxian Li) from Shanghai University, Anhui Polytechnic University, The University of Sydney, University of New South Wales, and Fuzhou University have delved into the preparation strategies and applications of HEM catalysts for electrochemical water electrolysis. The paper is published in the journal Frontiers in Energy.
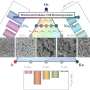
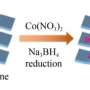
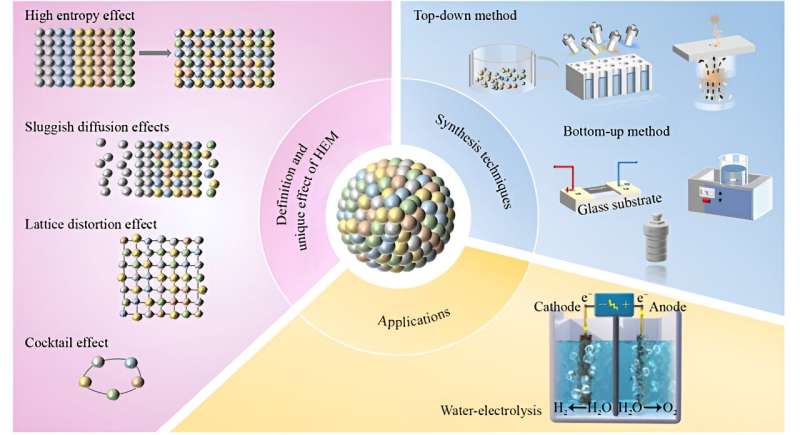
The study systematically reviews synthetic strategies for HEM catalysts, categorized into bottom-up and top-down approaches. The team's research focuses on the stabilization of HEMs, their catalytic mechanisms, and their application in supporting green hydrogen production.
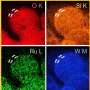
The findings reveal that HEM catalysts, composed of multiple elements, exhibit rich active sites and enhanced entropy stability. The team discovered that HEMs, with their unique high-entropy effect, lattice distortion effect, hysteresis diffusion effect, and cocktail effect, demonstrate excellent activity and stability.
These materials have shown the potential to become state-of-the-art catalysts for water electrolysis, with low energy consumption and extended service life. Specifically, nanoscale HEMs have been found to exhibit a larger specific surface area and stronger adsorption capacity, making them highly favorable for catalytic applications.
This research progress is expected to facilitate a deeper understanding of the basic properties of HEMs and the exploration of their electrocatalytic applications. The development of HEM-based catalysts for water electrolysis could significantly accelerate the design of low-cost, high-performance catalysts, thereby contributing to the advancement of clean energy generation and supporting the hydrogen economy.
The study's comprehensive review and analysis of HEMs in electrochemical water electrolysis underscore their potential to revolutionize the field of catalysis, offering a sustainable and efficient solution to the pressing need for clean energy production.
More information: Simiao Sha et al, High-entropy catalysts for electrochemical water-electrolysis of hydrogen evolution and oxygen evolution reactions, Frontiers in Energy (2023). DOI: 10.1007/s11708-023-0892-6
Provided by Shanghai Jiao Tong University Journal Center