This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Bifunctional CoFeP-N nanowires synthesized for sustainable water splitting
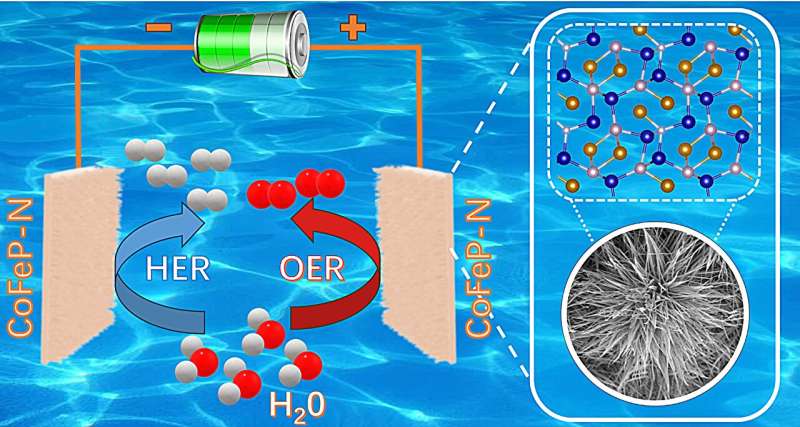
Prof. Wang Qi's research group from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences has synthesized iron- and nitrogen-co-doped CoFeP-N nanowires for high-efficiency electrocatalytic water splitting.
Their results, published in Applied Catalysis B: Environment and Energy, demonstrate the synthesis of bifunctional CoFeP-N nanowires for both hydrogen and oxygen evolution.
Hydrogen production by electrolysis uses water as the only raw material to achieve a closed cycle of hydrogen gas with zero carbon emissions, which is considered to be the greenest and most sustainable method. However, high costs limit the widespread use of electrolytic hydrogen production and require more cost-effective and efficient catalysts.
Due to their low cost and high catalytic performance, transition metal-based nanomaterials, which are abundant on Earth, have been shown to have broad prospects as excellent electrocatalysts.
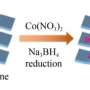
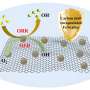
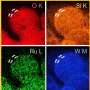
In this study, the researchers introduced various heteroatoms into the carrier to form a transition metal-based nanocomposite using a three-step synthesis method of hydrothermal phosphatization and low-temperature plasma treatment. They prepared bifocal CoFeP-N nanowires for hydrogen and oxygen evolution to achieve synergistic interactions with the catalyst.
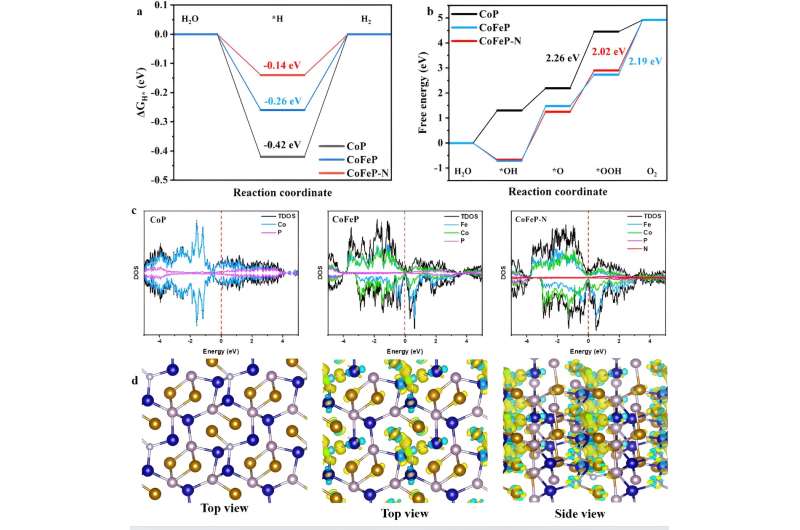
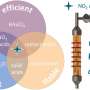
They used doping engineering, interface engineering, and plasma treatment to make the performance of transition metal catalysts potentially surpass precious metal catalysts, while maintaining good cycling stability. This helps to reduce production costs and promote industrial upgrading.
After CoFeP-N catalyst is prepared in an electrolysis cell, its electrocatalytic water splitting performance can exceed that of commercial precious metal electrolysis cells under the same conditions. In addition, it can work continuously for more than 100 hours without obvious performance degradation.
This work demonstrates an effective method for the preparation of transition metal-based bifunctional electrocatalysts, opening new avenues for the production of efficient, stable, and affordable advanced and sustainable energy materials.
More information: Ruiqi Wang et al, Low-temperature plasma-assisted synthesis of iron and nitrogen co-doped CoFeP-N nanowires for high-efficiency electrocatalytic water splitting, Applied Catalysis B: Environment and Energy (2024). DOI: 10.1016/j.apcatb.2024.124027
Provided by Chinese Academy of Sciences