This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New method could cut waste from drug production
Scientists have developed a sustainable new way of making complex molecules, which could greatly reduce waste produced during drug manufacturing, a study suggests.
The method—which is up to twice as efficient as previous techniques—is a new approach to a process that is key to guarding against the production of drugs with potentially disastrous side effects.
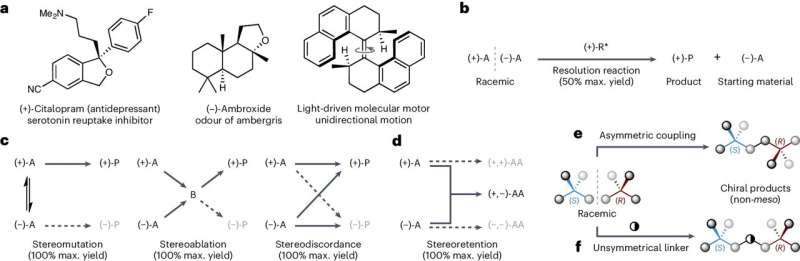
The team's insights could help prevent severe side effects caused by drugs that can exist in two mirror-image forms—left- and right-handed versions—such as the famous and tragic example of thalidomide, which was administered to pregnant women in the 1950s.
One form of thalidomide has the intended sedative effect, but the opposite mirror-image form interferes with fetal development. Many babies whose mothers were given the drug during pregnancy were born with severe birth defects.
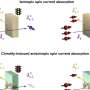
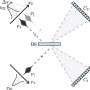
Now, researchers from the School of Chemistry have developed a new method to ensure only the left-handed or right-handed version of so-called chiral molecules is made—a process called asymmetric synthesis.
The team's technique works by bonding mixtures of left-handed and right-handed versions of a starting molecule together to produce a single-handed form of a target chemical.
The approach can produce target molecules in yields of up to 100 percent, meaning that theoretically, 100 target molecules are produced for every 100 starting molecules added, the team says.
This is far more efficient than many traditional methods, which are often limited to a maximum yield of just 50 percent, they add.
"Our work overturns the previously universally accepted limitation in what types of chiral molecules can be used as the starting materials in asymmetric synthesis. I am excited to see how this approach will be adopted in the future, particularly in the production of important bioactive compounds and functional organic materials," says Dr. David Jones of University College Cork, who was involved in the work while working at the University of Edinburgh.
The work could pave the way for a major expansion in scientists' ability to conduct asymmetric synthesis. It has the potential to impact various fields of science and technology in which the 3D shape of molecules is key to their function, the team says.
The importance of the creation of single-handed molecules—which include many drugs and chemicals used in agriculture—is widely recognized, with developments in the area twice winning the Nobel Prize in Chemistry in the past 20 years.
"Our new approach is the result of seven years of hard work by an incredibly talented group of international researchers. This has been possible thanks to European and UK agencies funding curiosity-driven, blue-skies research. This type of fundamental research is an essential component of what is required to ensure we can develop more sustainable chemical industries," says Professor Andrew Lawrence of the University of Edinburgh.
The research is published in the journal Nature Chemistry.
More information: Steven H. Bennett et al, Stereoretentive enantioconvergent reactions, Nature Chemistry (2024). DOI: 10.1038/s41557-024-01504-1
Journal information: Nature Chemistry
Provided by University of Edinburgh