August 29, 2016 report
Isolation of Fe(IV) decamethylferrocene salts
(Phys.org)—Ferrocene is the model compound that students often learn when they are introduced to organometallic chemistry. It has an iron center that is coordinated to the π electrons in two cyclopentadienyl rings. (C5H5- or Cp). Ferrocene is often used as a standard in electrochemical experiments because of its characteristic reversible oxidation of Fe(II) to Fe(III).
Due to its instability, very little research has been done to characterize Fe(IV) compounds. Researchers from Free University Berlin and Fredrich-Alexander-University Erlangen-Nürnberg have demonstrated that pentamethylcyclopentadienide (C5Me5- or Cp*) acts as a strong π-donating ligand that creates a stable Fe(IV)-containing compound. This ligand may serve as a model for investigating higher oxidation states for other metals. This work is published the recent issue of Science.
In most cases in which researchers have isolated a compound with an Fe(IV) oxidation state, the metal was coordinated to ligands that offer strong π-donor properties such as terminal nitrido and oxido ligands. These compounds are typically unstable and difficult to analyze. Malischewski, et al. were able to isolate the [Cp*2Fe]2+ dication as a salt with several different anions, allowing them to conduct crystal studies as well as electronic studies using SQUID and Mössbauer spectroscopy.
To make the Fe(IV) organometallic salts, [Cp*2Fe] was combined with strong oxidizers (AsF5, SbF5, ReF6, [XeF](Sb2F11)) in either sulfur dioxide or hydrogen fluoride. The resulting salts were isolable and analyzed.
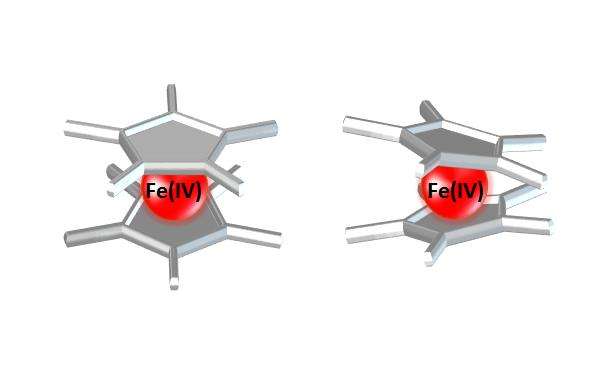
The crystal structures of each of the salts showed slight differences in the way the Cp* ring was tilted, which the authors attribute to the identity of the counterion. The rings displayed no tilt with Sb2F11- and tilts ranging from 4.17o for As2F11- to 16.56o for SbF6-. The large anions with eleven fluorides seem to promote little ring tilt while the smaller anions with six fluorides promoted a more pronounced tilt. This may be due the stronger interactions of the polarized cation with the smaller anions, which possess higher charge densities.
All of the structures showed hydrogen bonding between methyl and fluorine and a large distance between fluorine and iron. The hydrogen bonding between the methyl and fluorine is more pronounced in the smaller anions and may be another contributing factor to the observed ring tilting. Notably, the Fe-C bond lengths were much longer than the Fe-C bonds in its lower oxidation states.
SQUID studies showed that the compounds possess effective magnetic moments of slightly over 3 µB, indicative of two unpaired electrons. The temperature dependencies of the magnetic moments further suggest the presence of considerable spin-orbit interactions. Additionally, Mössbauer spectroscopy studies correlate with the obtained structural parameters and indicate an oxidation state of +4. Both techniques suggest a d-orbital ordering similar to that of ferrocene. This means that the ordering changes two times in a stepwise fashion going from Fe(II) to Fe(IV).
This research provides interesting insights into a stable Fe(IV) metallocene. Further research may include possible applications of [Cp*2Fe]2+ salts as well as studying other metals with multiple oxidation states.
More information: M. Malischewski et al. Isolation and structural and electronic characterization of salts of the decamethylferrocene dication, Science (2016). DOI: 10.1126/science.aaf6362
Abstract
Ferrocene and its decamethyl derivative [Cp*2Fe] are the most common standards for nonaqueous electrochemical investigations because of their well-defined and only mildly solvent-dependent reversible Fe(II)/Fe(III) redox couple. Higher oxidation states have only rarely been studied. We report the isolation and crystallographic and spectroscopic characterization of surprisingly stable Fe(IV) salts of the [Cp*2Fe]2+ dication, produced by oxidation of [Cp*2Fe] with AsF5, SbF5, or ReF6 in neat sulfur dioxide as well as [XeF](Sb2F11) in neat hydrogen fluoride. The Sb2F11– salt exhibits a metallocene with the expected mutually parallel arrangements of the Cp* rings, whereas the As2F11–, AsF6–, SbF6–, and ReF6– salts manifest tilt angles ranging from 4° to 17°. Both 57Fe Mössbauer spectroscopy and superconducting quantum interference device magnetization studies reveal identical d-orbital splitting with an S = 1, 3E ground state based on the 3d electronic configuration e2g3a1g1 of all [Cp*2Fe]2+ salts.
Journal information: Science
© 2016 Phys.org