November 17, 2015 report
Functionalized porous electrode used for radioactive waste product
(Phys.org)—One of the concerns with using nuclear energy is the management of the waste produced. Much of reactor waste can be recycled, however one byproduct, americium-241 is radioactive, and is responsible for a large proportion of the heat generated. It is difficult to separate from the recyclable waste.
Am(III), americium's stable oxidation state in water and acidic solutions, is difficult to remove using a ligand complex because its coordination chemistry is very similar to the other actinides and lanthanides. While higher oxidation states would allow separation and removal using solvent extraction methods, oxidizing Am(III) requires prohibitively high potentials. Oxidation has been achieved previously by others using strong chemical oxidants, however, a useful electrochemical method to make high oxidation state americium for nuclear waste processing has eluded scientists for more than half a century.
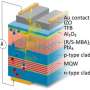
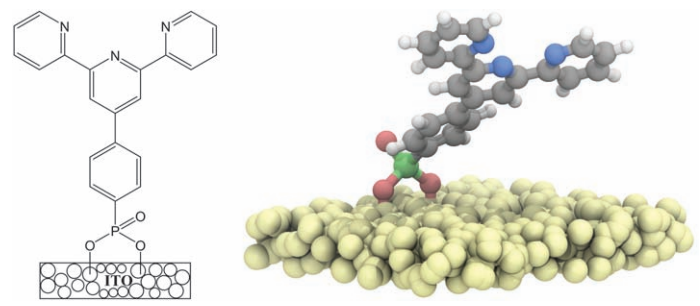
Christopher J. Dares, Alexander M. Lapides, Thomas J. Meyer from the University of North Carolina at Chapel Hill, and Bruce J. Mincher from Idaho National Laboratory showed that by modifying a highly porous nanoparticle electrode with p-tpy, a ligand that is known to coordinate with Am(III), they are able to lower the oxidation potentials for Am(III) to Am(V) and Am(VI) in acidic solution and therefore separate americium from other nuclear waste products.
When oxidized to Am(V) and Am(VI), americium forms the oxo complexes [AmO2]2+ and [AmO2]+. They are easier to remove from waste streams than Am(III), but to exploit them requires overcoming the high potential for oxidizing Am(III) to Am(IV) (more than 1 volt beyond the potential required to oxidize water to oxygen). Based on previous studies involving the surface modification of oxide electrodes, Dares, et al. investigated mesoporous oxide electrodes and the influence of ligand binding on the oxidation of metal ions in the external solution.
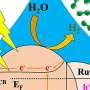
Dares, et al. covered their tin-doped indium oxide (ITO) electrode surface with p-tpy, a known ligand for metal ion binding, and monitored Am(III) conversion to Am(V) and Am(VI) during electrolysis using spectroscopic measurements. They conducted their spectroscopic measurements every three minutes during the electrolysis of a 0.43 mM solution of 243Am(III) at 1.8 V (vs. SCE in 0.1 M nitric acid). They observed formation of the higher oxidation states of americium below the 2.6 V potential required to oxidize Am(III) to Am(IV), with evidence for the importance of an Am(III)-p-tpy complex on the surface in decreasing the thermodynamic barrier for oxidation of Am(III) to Am(IV) which triggers further oxidation to [AmO2]2+ and [AmO2]+.
On a first run, half of the Am(III) had converted to Am(V), but no Am(VI) formed, likely due to a competing autoreduction reaction caused by the effects of the radioactive decay of the americium. After adjusting the concentration of Am(V), Dares et al. demonstrated that Am(VI) can be generated electrochemically at 1.8 V. The mechanism appears to involve surface binding of Am(III) and oxidation to Am(IV) followed by further oxidation to Am(V) and release as [AmO2]+.
Radioactive decay of americium results in the release of high energy radiation which is absorbed by water in a process known as radiolysis. One of the products of water radiolysis is hydrogen peroxide and it reduces Am(VI) making it unstable. Dares, et al. conducted an electrolysis study in which they varied the applied potential to study the competitive effects of electrochemical oxidation, and radiolytically induced reduction in aqueous acidic solutions.
This study shows that one possible route to removing americium-241 from nuclear waste is through electrochemical oxidation to higher-order oxidation states. While normally prohibitive because of the high potential required, by using a ligand-functionalized mesoporous electrode, the oxidation potential of Am(III) to Am(IV) is decreased, opening a route to [AmO2]2+ that will be much easier to separate from nuclear waste.
More information: C. J. Dares et al. Electrochemical oxidation of 243Am(III) in nitric acid by a terpyridyl-derivatized electrode, Science (2015). DOI: 10.1126/science.aac9217
ABSTRACT
Selective oxidation of trivalent americium (Am) could facilitate its separation from lanthanides in nuclear waste streams. Here, we report the application of a high-surface-area, tin-doped indium oxide electrode surface-derivatized with a terpyridine ligand to the oxidation of Am(III) to Am(V) and Am(VI) in nitric acid. Potentials as low as 1.8 volts (V) versus the saturated calomel electrode were applied, 0.7 V lower than the 2.6 V potential for one-electron oxidation of Am(III) to Am(IV) in 1 molar acid. This simple electrochemical procedure provides a method to access the higher oxidation states of Am in noncomplexing media for the study of the associated coordination chemistry and, more important, for more efficient separation protocols.
Journal information: Science
© 2015 Phys.org