June 14, 2016 report
New xenon oxides may provide clues to the missing xenon paradox
(Phys.org)—Xenon, one of the noble gases, forms oxides that are not stable under ambient conditions, but are stable under very high pressure. In an effort to understand why the Earth's crust and atmosphere lacks the expected amount of xenon, researchers from institutions in France, the U.K. and Japan have made two new xenon compounds under high pressure conditions.
The first compound Xe3O2 was predicted by another group, but was experimentally verified by Agnes Dewaele, Nicholas Worth, Chris J. Pickard, Richard J. Needs, Sakura Pascarelli, Oliver Mathon, Mohamed Mezouar and Tetsuo Irifune. The other compound, Xe2O5, was a newly discovered compound by this group. Their research appears in Nature Chemistry.
Compared to its fellow noble gases, argon and krypton, there is relatively little xenon in the Earth's atmosphere. This goes against predictions based on the relative abundance of xenon in carbonaceous chondrites, meteorites that are thought to be made of the same materials as the early Earth. This difference between expected and actual abundance of xenon is known as the "missing xenon paradox."
One theory to account for the missing xenon is that it is located within the Earth's lower mantle or the core. The Earth's core would have a very different reaction conditions from the crust. One difference would be the extremely high pressures. Xenon chemistry changes at high pressure. At pressures above 80 GPa xenon takes on a hexagonal close-packed morphology. At around 135 GPa, xenon has a metallic character. Xenon may be trapped within the mantle as an inorganic compound.
Dewaele, et al. wanted to investigate xenon oxides under high pressure since oxygen is the most abundant element in the Earth's mantle. Xe3O2 was predicted by Hermann and Schwerdtfeger to be stable at pressures above 75GPa. Using laser-heated diamond anvil cells containing Xe and O2, Dewaele, et al. were able to experimentally verify the theoretical compound at pressures around 97 GPa.
Xe3O2 was made with an oxygen concentration of less than 25mol% and has a three-dimensional morphology that consists of planar chains of XeO4 squares. Xenon atoms within the chains likely have a Xe4+ oxidation state while between the planar chains are unoxidized xenon atoms. Xe3O2 was stable to about 38 GPa.
Additionally using the same methods but in a high-O2 environment (O2 levels above 50mol%), Dewaele, et al. also isolated Xe2O5, a new xenon compound. It was stable at pressures above 77 GPa. Both compounds were characterized using powder x-ray diffraction, x-ray absorption spectroscopy, and Raman spectroscopy.
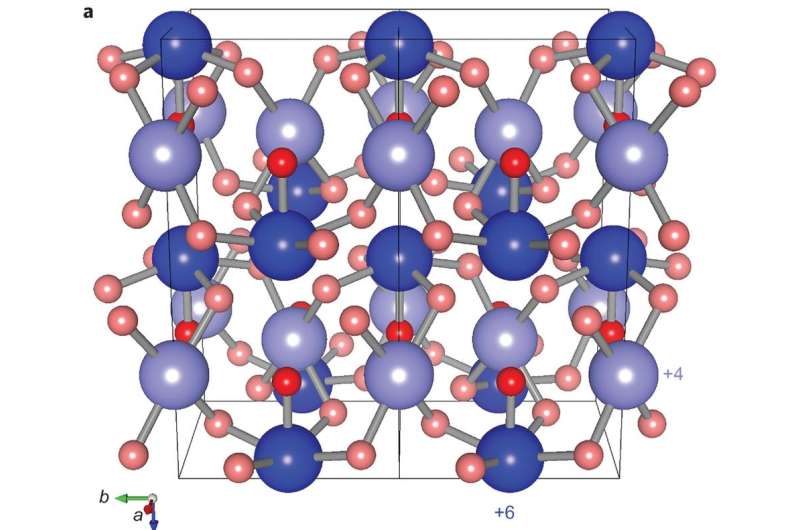
The experimental data on crystal structure of Xe2O5 were used to constrain theoretical stretches of a stable xenon oxide. This was how a Xe2O5 formula and structure with a minimal enthalpy were identified. In this structure, each xenon atom is connected to an oxygen atom with square planar corners, and every other xenon bonds with a fifth oxygen atom to form a square pyramid. Notably these two xenon atom types are different oxidation states, Xe4+ and Xe6+, and the Xe-O bond remains intact as the pressure is lowered.
Additional studies looking at the highest occupied molecular orbitals at pressure around 83 GPa showed that Xe2O5 would likely be an insulator, while Xe3O2 has properties indicative of a small bandgap semiconductor.
This research provides experimental and theoretical justification for two new xenon oxide compounds whose minimal pressure at which they are stable is lower than previously thought, indicating that these xenon oxides are more stable than predicted. According to Dr. Dewaele, these studies suggest that xenon's geochemical definitions may need to be revised to account for its different reactivity under high pressure.
More information: Agnès Dewaele et al. Synthesis and stability of xenon oxides Xe2O5 and Xe3O2 under pressure, Nature Chemistry (2016). DOI: 10.1038/nchem.2528
Abstract
The noble gases are the most inert group of the periodic table, but their reactivity increases with pressure. Diamond-anvil-cell experiments and ab initio modelling have been used to investigate a possible direct reaction between xenon and oxygen at high pressures. We have now synthesized two oxides below 100 GPa (Xe2O5 under oxygen-rich conditions, and Xe3O2 under oxygen-poor conditions), which shows that xenon is more reactive under pressure than predicted previously. Xe2O5 was observed using X-ray diffraction methods, its structure identified through ab initio random structure searching and confirmed using X-ray absorption and Raman spectroscopies. The experiments confirm the recent prediction of Xe3O2 as a stable xenon oxide under high pressure. Xenon atoms adopt mixed oxidation states of 0 and +4 in Xe3O2 and +4 and +6 in Xe2O5. Xe3O2 and Xe2O5 form extended networks that incorporate oxygen-sharing XeO4 squares, and Xe2O5 additionally incorporates oxygen-sharing XeO5 pyramids. Other xenon oxides (XeO2, XeO3) are expected to form at higher pressures.
Journal information: Nature Chemistry
© 2016 Phys.org