Measurements in the nanoworld: A carbon 'eye' monitors changes in pH close to molecules
Usually, measuring the pH of a solution is not a problem. But how can researchers examine changes in acidity or alkalinity occurring at the nanoscale, for example, at a surface showing the very first signs of pitting corrosion? A novel method of pH measurement at the nanoscale has just been presented by scientists from the Institute of Physical Chemistry of the Polish Academy of Sciences in Warsaw.
At Warsaw's Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) a nanosensor has been developed for the continuous monitoring of changes in pH. Used as a scanning electrochemical microscope probe, it allows for the precise measurement of changes in acidity/alkalinity occurring over very small fragments of the surface of a sample immersed in a solution. The spatial resolution here is just 50 nm, and in the future, it can be reduced even further.
"The ability to monitor changes in the acidity or alkalinity of solutions at the nanoscale, and thus over areas whose dimensions can be counted in billionths of a metre, is an important step toward better understanding of many chemical processes. The most obvious examples here are various kinds of catalytic reactions or pitting corrosion, which begins on very small fragments of a surface," explains Prof. Marcin Opallo (IPC PAS).
Changes in the pH of solutions are due to reactions involving hydrogen ions (i.e. positively charged protons, H+) or hydroxide ions (negatively charged, OH-). These reactions interfere with the ratio between the numbers of ions of both types. The more protons there are, the more acidic the environment is; the more hydroxide ions, the more alkaline. The pH indicates the scale of the disturbance of neutrality. Its values are generally within the rangle of zero to 14, where in a neutral environment the value is seven; a pH of less than seven corresponds to acids, and higher values to alkalis.
Measuring the pH of solutions is not generally difficult and part of standard laboratory and industrial practice. However, measuring changes in extremely small volumes—for example, directly in the vicinity of individual chemical molecules—is more difficult. To date, there are no instruments capable of detecting changes in pH at such a small, nanometric scale.

"With our nanosensor we can measure the pH with a resolution similar to the diameter of the carbon electrode scanning over the surface of the sample, which is currently 50 nm. We determine the pH in the range of two to 12 to two decimal places, as well as being able to keep track of its changes, even dozens of times per second," says Dr. Wojciech Nogala (IPC PAS), one of the inventors.
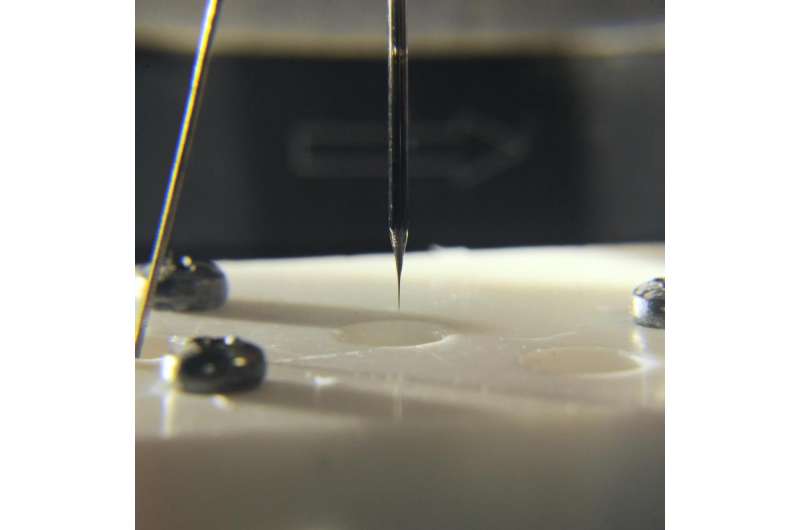
The preparation of the carbon nanoelectrode starts by heating up a thin quartz tube with a laser beam. At an appropriately selected temperature, quartz becomes malleable and the tube can be gently stretched, which results in reduced diameter. Finally, the process leads to the formation of a glass 'hair' with an outer diameter of approximately 100 nm and an inner tunnel with a diameter of approximately 50 nm. This pipette is then surrounded by an argon atmosphere. Next, a mixture of propane and butane is introduced into the pipette and pyrolysis occurs, i.e. decomposition at a high temperature. Carbon is progressively deposited on the inner walls of the pipette (the lack of oxygen prevents it from burning). Conducted carefully and for long enough, the pyrolysis completely fills the interior of the nanopipette with carbon, making it into a long and extremely thin rod.
"The method of production of ultra-thin carbon electrodes has been known for several years and is in use in a few research centres in the world. However, an electrode obtained in this manner is not suitable for measuring pH. We had to modify it with something that would react with changes in the concentration of protons and hydroxide ions. To this end, we used a compound known as syringaldazine," explains Ph.D. student Magdalena Michalak (IPC PAS), the first author of the publication in the journal Analytical Chemistry, in which the construction of the nanosensor is described.
The syringaldazine molecule can be electrochemically oxidized, resulting in the elimination of two electrons and two protons. Since the reaction is reversible, oxidized syringaldazine can be reduced to its original form by adding on two protons and two electrons from the environment. For the IPC PAS researchers, what was most important was the fact that the redox potential of the oxidation/reduction of syringaldazine depends on the pH of the solution and varies by 59 millivolts per pH unit. After the deposition of syringaldazine at the end of the carbon electrode, it is possible to carry out pH measurement by monitoring changes in the flow of current depending on the applied voltage.
"It's easy to say, a little harder to do. We are talking about measuring picoamperes. Why, even the currents induced in the antenna of a radio receiver are a thousand times larger! So we have to carry out the measurements of pH changes inside a Faraday cage, which isolates the sensor and sample from the electromagnetic fields that are all around us," says Dr. Nogala.
The pH mapping using the modified carbon nanoelectrode used SECMx software prepared by Prof. Gunther Wittstock from the University of Oldenburg.
The long-term goal of the research at the IPC PAS is to develop methods for monitoring pH changes taking place in the vicinity of individual chemical molecules. Such sophisticated measurements would make it possible to, for example, resolve in what manner enzyme molecules lose their activity – whether the process occurs gradually in each molecule in the solution simultaneously, or whether individual molecules lose their activity suddenly.
More information: "Voltammetric pH Nanosensor"; M. Michalak, M. Kurel, J. Jedraszko, D. Toczydlowska, G. Wittstock, M. Opallo, W. Nogala; Analytical Chemistry, 2015 Dec; 87(23):11641-5. DOI: 10.1021/acs.analchem.5b03482
Journal information: Analytical Chemistry
Provided by Polish Academy of Sciences