Metal-free recyclable polyester produced from a biomass-derived compound
Most plastic and polymer materials around are poorly sustainable, as they are petroleum-based, their preparation needs metal catalysis, and they persist in the environment and cannot be effectively recycled. Seeking for true sustainability, American scientists have now prepared a fully recyclable biopolyester from a biofeedstock-based chemical without any metal catalyst involved. As they report in the journal Angewandte Chemie, the polymer is best suited for regular polyester applications, and its monomer component is completely recovered upon heating at high temperatures.
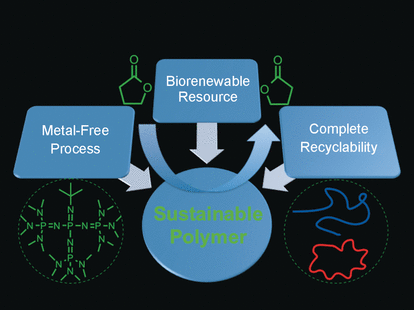
Sustainability and recyclability are key requirements for next-generation polymers. Furthermore, atom economy during the preparation process is also requested because it is desirable to produce as little waste as possible. One possible candidate for such atom-economic polymerization to form a recyclable biopolymer was the succinic acid derived biochemical gamma-butyrolactone or GBL, but its polymerization to poly-GBL or PGBL turned out to be extremely challenging and could only be achieved under very harsh conditions and metal catalysis. Now, Eugene Y.-X. Chen and his postdoctoral fellow Miao Hong at Colorado State University have identified a purely organocatalytic approach: "For biomedical applications, it would be desirable that PGBL could be produced by metal-free organopolymerization of GBL," Chen describes their motivation.
Their challenge was to push forward a reaction that is deemed thermodynamically unfavorable. Thus, the scientists employed an extraordinarily strong organic base to make the molecule more reactive and initiate an atom-economic procedure, which is called ring-opening polymerization. As a result, at temperatures as low as −40 °C and ambient pressure, the reaction proceeded smoothly. "This system enabled high monomer conversion of up to 90 % and high-molecular-weight polymers in a relatively short time period of four hours or less," the scientists write. They obtained the polymer product as a powder, which displayed the typical properties of a polyester material that can be cast into different forms and shapes.
Its most important property, however, is its recyclability. The authors emphasize: "The PGBL obtained by the current organopolymerization is completely recyclable back to its monomer in the pure state upon heating the bulk polymer." And as no metal catalysis is involved, this process will be especially attractive for applications where metal-free products or processes are of major concern. Therefore its main targets are the biomedical or microelectronics industry.
More information: Miao Hong et al. Towards Truly Sustainable Polymers: A Metal-Free Recyclable Polyester from Biorenewable Non-Strained γ-Butyrolactone, Angewandte Chemie International Edition (2016). DOI: 10.1002/anie.201601092
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Angewandte Chemie