November 30, 2015 feature
New membrane improves energy harvesting by reverse electrodialysis
(Phys.org)—Researchers have constructed a new type of nanoporous membrane that does an exceptionally good job at selectively controlling ion transport—for instance, allowing negatively charged ions to pass through the pores, while prohibiting the passage of positively charged ions. To demonstrate one possible application, the researchers developed the membrane into an energy conversion device that harvests energy using its ability to separate charged particles. The technique is very similar to reverse electrodialysis, but the membrane's structure eliminates one of the limitations of traditional reverse electrodialysis, resulting in increased power generation.
The scientists, Zhen Zhang and Liping Wen from the Chinese Academy of Sciences and their coauthors, have published a paper on the new membrane in a recent issue of the Journal of the American Chemical Society.
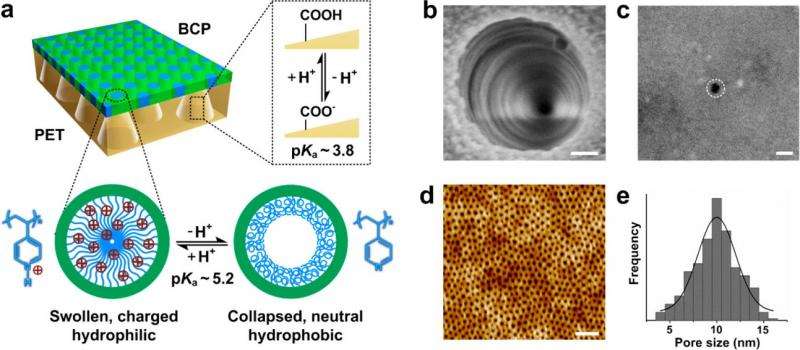
The researchers describe the new membrane as an "engineered asymmetric heterogeneous membrane": "engineered" because it is a robust version of the fragile cell membranes used in living organisms, "asymmetric" because each side of the membrane filters the ions differently, and "heterogeneous" because each side is composed of a different material. Here, the researchers used two types of polymer materials: block copolymer (BCP) and polyethylene terephthalate (PET). Although not all asymmetric membranes are heterogeneous, heterogeneous ones have certain advantages such as being easier to fabricate and offering greater multifunctionality than asymmetric membranes made of a single material.
The greatest feature of the new membrane is that it promotes ion transport in one direction and inhibits it in the other direction. As a result, a much larger current is generated in one direction across the membrane than in the opposite direction, which is called "ionic current rectification." The new membrane has a rectification ratio of about 1075, which is more than twice the highest ratio reported to date. As the rectification ratio represents an asymmetric flow of charge, it arises from asymmetries in the new membrane (including chemical, geometrical, and charge asymmetries), and is highly desirable for various applications.
"This behavior is similar to the mechanism of semiconductor diodes for controlling the transport of electrons, and it represents the ability of directionally delivering specific types and controllable amounts of molecules or ions," Wen told Phys.org.
"It will open a new way to handle molecular or ion species in fluid and show broad application prospects in many fields. Similar to the semiconductor electronic circuits, the nanofluidic diode with a high rectification ratio represents the key building block for ionic circuits, which would allow for regulating, sensing, concentrating, and separating ions and molecules in electrolyte solutions. Also, in asymmetric-membrane-based photoelectric energy conversion systems, a high rectification ratio is favored, as the opposite transmembrane ionic transport will suppress the power density."
As an energy conversion device, the new membrane functions similar to reverse electrodialysis, in which energy can be harvested from the differences in the ion concentration (for example, negatively charged chlorine ions and positively charged potassium ions) on opposite sides of the membrane. Under a concentration gradient, the chlorine ions spontaneously diffuse across the membrane in order to lower the concentration gradient, and the energy generated by the current produced by the ion diffusion can be harvested.
The researchers predict that the new membrane can produce a power output of thousands of watts per square meter, which is equivalent to and potentially exceeding that generated by some commercially available reverse electrodialysis membranes. When investigating the reason for this high performance, the researchers found that the new membrane's asymmetries allow it to eliminate the "concentration polarization" problem that hinders traditional reverse electrodialysis membranes. The problem is that, on the side of the membrane with the low concentration of chlorine ions, the chlorine ions tend to clump together at the positively charged pore openings. This clumping creates a higher concentration of chlorine ions near the pore openings than in the bulk solution, which makes the concentration difference across the membrane seem lower than it actually is, causing fewer chlorine ions to diffuse across the membrane.
The new membrane not only eliminates this problem, but it actually make the chlorine ion concentration near the pore openings lower than in the bulk concentration, which promotes ion diffusion. The researchers attribute this advantage to the membrane's asymmetric structure and slightly negatively charged pores, in contrast to the symmetric structure and positively charged pore openings of traditional membranes.
"When the new developed membrane is applied in salinity gradient power generation, the output power density can be increased considerably due to the fact that the concentration polarization phenomenon that commonly exists in traditional reverse electrodialysis can be eliminated using the asymmetric bipolar structure, which provides guidance on the current ion-exchange-membrane-based energy conversion systems," Wen said.
In the future, the researchers hope to further improve the membranes so that they can be used for applications including power generation, water purification, and desalination.
"We would like to develop ionic diode membranes with more powerful functions by optimizing the composition of the selective membrane, increasing the porosity, and reducing the cost, which will finally meet the requirements for real-world applications," Wen said.
More information: Zhen Zhang, et al. "Engineered Asymmetric Heterogeneous Membrane: A Concentration-Gradient-Driven Energy Harvesting Device." J. Am. Chem. Soc., Article ASAP. DOI: 10.1021/jacs.5b09918
Journal information: Journal of the American Chemical Society
© 2015 Phys.org