September 22, 2015 feature
'Golden' silver nanoparticle looks and behaves like gold
(Phys.org)—In an act of "nano-alchemy," scientists have synthesized a silver (Ag) nanocluster that is virtually identical to a gold (Au) nanocluster. On the outside, the silver nanocluster has a golden yellow color, and on the inside, its chemical structure and properties also closely mimic those of its gold counterpart. The work shows that it may be possible to create silver nanoparticles that look and behave like gold despite underlying differences between the two elements, and could lead to creating similar analogues between other pairs of elements.
The researchers, led by Osman Bakr, Associate Professor of Materials Science and Engineering at King Abdullah University of Science and Technology (KAUST) in Saudi Arabia, have published the paper in a recent issue of the Journal of the American Chemical Society.
"In some aspects, this is very similar to alchemy, but we call it 'nano-alchemy,'" Bakr told Phys.org. "When we first encountered the optical spectrum of the silver nanocluster, we thought that we may have inadvertently switched the chemical reagents for silver with gold, and ended up with gold nanoparticles instead. But repeated synthesis and measurements proved that the clusters were indeed silver and yet show properties akin to gold. It was really surprising to us as scientists to find not only similarities in the color and optical properties, but also the X-ray structure."
Like all chemical elements, silver and gold are defined by their number of protons: silver has 47, and gold has 79. The work here doesn't change the number of protons in an atom of silver; otherwise it would no longer be considered silver. Instead, the researchers synthesized a nanocluster of 25 silver atoms, along with 18 other molecules called "ligands" that surround the silver atoms. The entire negatively charged, silver-based complex ion has the chemical formula [Ag25(SPhMe2)18]-.
Although a few other silver nanoclusters have been synthesized in recent years, this is the first silver nanocluster that has a matching analogue in gold: [Au25(SPhMe2)18]- has previously been reported. Besides both nanoclusters having 25 metal atoms and 18 ligands, they also both have all of their atoms and electrons arranged in almost exactly the same way.
In their study, the researchers performed tests demonstrating that the silver and gold nanoclusters have very similar optical properties. Typically, silver nanoclusters are brown or red in color, but this one looks just like gold because it emits light at almost the same wavelength (around 675 nm) as gold. The golden color can be explained by the fact that both nanoclusters have virtually identical crystal structures.
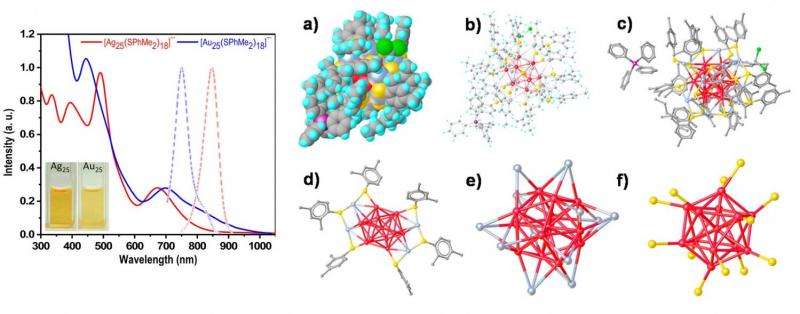
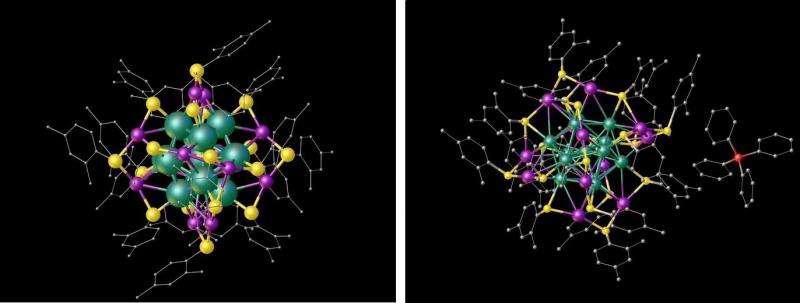
The scientists investigated the silver nanocluster's crystal structure using X-ray diffraction, in which an X-ray beam strikes the crystallized structure and is reflected at various angles to create a diffraction pattern on a detector. This technique revealed that the silver nanocluster has one silver atom at the center of a 12-pointed-star-like shape called an icosahedron. While 12 of the other silver atoms form the 12 points, the remaining 12 silver atoms occupy some of the faces. This arrangement is almost exactly like that of the gold nanocluster, except that three of the atoms on the faces of the silver nanocluster are turned in a different direction. As far as the scientists can tell, the orientation of these three atoms is the only notable structural difference between the silver and gold nanoclusters, and it causes a slight distortion in the silver nanoclusters.
The question naturally arises: why are these silver and gold nanoclusters so similar, when individual atoms of silver and gold are very different, in terms of their optical and structural properties? As Bakr explained, the answer may have to do with the fact that, although larger in size, the nanoclusters behave like "superatoms" in the sense that their electrons orbit the entire nanocluster as if it were a single giant atom. These superatomic orbitals in the silver and gold nanoclusters are very similar, and, in general, an atom's electron configuration contributes significantly to its properties.
"The size scale of nanoparticles lies in between atoms/molecules and bulk material, where the absolute rule of neither quantum nor classical physics is observed," Bakr explained. "However, the Ag nanoparticle we synthesized was so small in size that it actually behaves a lot like an atom, i.e., a superatom. Since the structural framework of Ag25 is nearly identical to Au25, which makes similar atomic arrangements in 3D space, this special atomic arrangement allows for the hybridization of Ag atomic orbitals and ligand orbitals (the organic molecules surrounding the metal) in Ag25 to produce superatomic orbitals that are very similar to the well-known Au25 system. This could be the main reason for the similarities observed between the Ag and Au clusters, which may not be possible to achieve with individual atoms or bulk materials."
While the results here show that silver can acquire the properties of gold, the reverse may also be possible, with gold being synthesized to look and behave like silver.
"If silver can acquire properties of gold, there is no obvious reason why the reverse shouldn't be possible," Bakr said.
This duality, in which one type of atom acquires the properties of another, has the potential to offer unprecedented abilities in nanoscience research, and is one area that the scientists plan to investigate more in the future.
The researchers also hope that the results will lead to a better understanding of the fundamental differences between gold and silver. For instance, although both materials are lustrous metals, gold is relatively biocompatible and being researched for biomedicine, whereas silver is cytotoxic and used in antibacterial surface coatings. Questions like these may be answered by blurring the lines between the elements as we know them.
"Our future plan is to synthesize other sizes of gold clusters and other metal analogues of gold nanoparticles to explore whether these clusters would still show the behavior of gold or not," Bakr said. "Our goal is to find cheaper substitutes for gold in applications where gold nanoparticles are required."
More information: Chakra P. Joshi, et al. "[Ag25(SR)18]-: The 'Golden' Silver Nanoparticle." Journal of the American Chemical Society. DOI: 10.1021/jacs.5b07088
Journal information: Journal of the American Chemical Society
© 2015 Phys.org