A 'frozen reaction' as key to eco-friendly chemical catalysis
Enzymes are naturally existing biocatalysts of great potential for application in sustainable chemistry. Yet, controlling enzyme reactions at atomic level is still a challenge in biology. Scientists at the Max Planck Institute for Terrestrial Microbiology provide novel tools to study enzyme catalysis, which allow them to direct the incorporation of 'naked hydrogen atoms' into substrates.
Chemical reactions require the controlled incorporation, removal or change of atoms and atomic bonds in molecules. Whereas chemists often need to use extreme conditions and toxic substances – such as organic solvents - for this task, Nature simply relies on enzymes. Enzymes are natural existing biocatalysts that are able to catalyze chemical reactions with high efficiency, high specificity and under mild, environmentally friendly conditions. This makes enzymes perfectly suited tools for a use in eco-friendly and sustainable processes.
To realize this vision, it is important to understand how enzymes work and how their reactivity can be controlled. The research group of Tobias Erb at the Max Planck Institute for Terrestrial Microbiology in Marburg investigates the basis of enzyme catalysis to functionalize them for synthetic biological processes. One of the most prevalent enzyme reactions in Nature is the transfer of hydrogen atoms onto molecules. Unfortunately, this reaction is so fast that it is almost impossible to study the corresponding enzymes in detail. To make exactly this possible, the research group of Erb in Marburg teamed up with scientist from ETH Zurich to develop a novel method to study single chemical steps inside of enzymes.
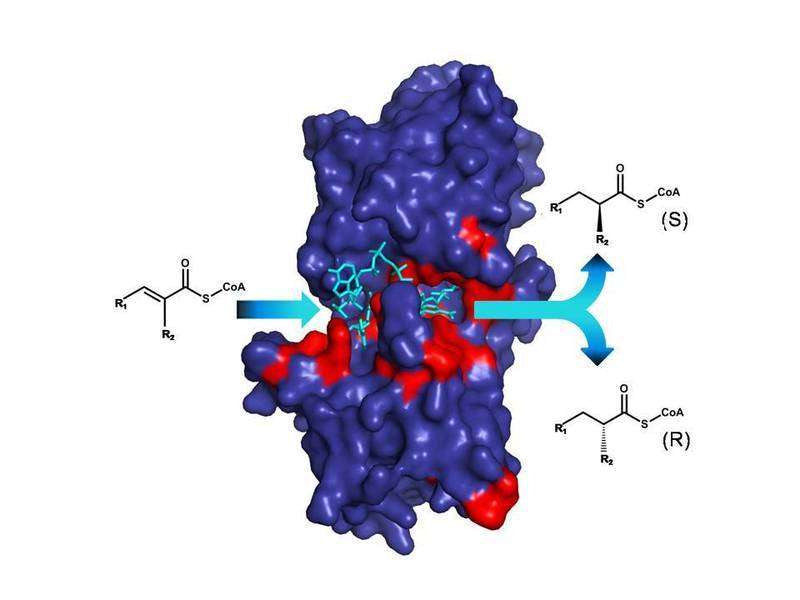
The basis for these experiments is a molecular probe that represents a 'frozen' chemical reaction. This molecular probe is activated inside of the enzyme, where it allows to measure only the last part of the enzyme reaction, the transfer of the 'naked hydrogen atom'. "So far, we knew that the enzymes we studies transfer naked hydrogen atoms, but we did not know, where they are coming from", explains Raoul Rosenthal, the first author of the study that just appeared in the journal Nature Chemical Biology. The molecular probe allowed the scientist to finally answer this longstanding question.
"With the new insight, we were then able to rationally design the enzyme reaction", says Bastian Vögeli one of the scientist from Marburg. To that end, the researchers changed the inside of the enzyme so that the naked hydrogen atom was incorporated from another side to the target molecule. "Our results demonstrate that we can generate novel products with these 'designer enzymes' in a targeted way", says Vögeli. The team of Tobias Erb is confident that their results will be important for future biotechnological applications. The fact that the enzymes studied play an important role in antibiotic biosynthesis gives the Max Planck scientists hope that by enzyme manipulation new antibiotically active molecules can be created in an eco-friendly manner, which will find their way into medicine and therapeutic applications.
More information: "The use of ene adducts to study and engineer enoyl-thioester reductases" Nature Chemical Biology (2015). DOI: 10.1038/nchembio.1794
Journal information: Nature Chemical Biology
Provided by Max Planck Society