Team pioneers strategy for creating new materials
Making something new is never easy. Scientists constantly theorize about new materials, but when the material is manufactured it doesn't always work as expected. To create a new strategy for designing materials, scientists at the Department of Energy's Argonne National Laboratory combined two different approaches at two different facilities to synthesize new materials.
This new strategy gives faster feedback on what growth schemes are best, thus shortening the timeframe to manufacture a new, stable material for energy transport and conversion applications.
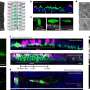
A recent article in Nature Materials describes how researchers used X-ray scattering during a process called molecular beam epitaxy (MBE) to observe the behavior of atoms as a type of material known as layered oxides were being formed. These observations were then used as data for computational predictions of new materials, leading to insights on how to best combine atoms to form new, stable structures.
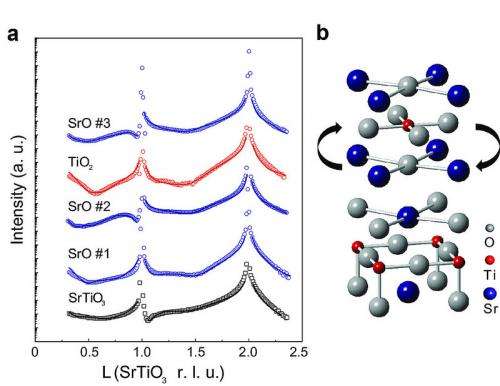
"MBE is the construction of new materials one layer at a time—and each layer is one-atom thick. We used a new type of MBE system to observe what happens during the growth of oxide thin films. We found that the layers spontaneously rearrange to reach a lower energy, preferred configuration—but not necessarily the configuration we intended," said John Freeland, the Argonne physicist who led the team. "Most scientists would not expect layers to move around like this, but this is important information to know when designing new materials."
In experimenting with a class of oxides known as strontium titanates, the research team found that when they layered titanium on top of two layers of Strontium, the titanium layer switched places with the second strontium layer, thus becoming the center layer. When titanium was layered on multiple layers of strontium, titanium always switched places with the strontium layer directly underneath it.
Argonne chemist June Hyuk Lee lead the experimental development of the in situ oxide MBE, and Guangfu Luo from the University of Wisconsin-Madison developed the theoretical approach to unraveling the energetics that drive the layer rearrangements.
The research team included expertise from Argonne's Advanced Photon Source (APS), Center for Nanoscale Materials (CNM), Chemical Sciences and Engineering, and Materials Science, and partners from Northwestern University, the University of Connecticut-Storrs and the University of Wisconsin-Madison, who wanted to understand the driving force behind the rearrangements. Using density functional theory (DFT) and computational resources at the CNM, they calculated and compared the energies of different layer sequences, using the data collected from the MBE system. They found that the actual layer sequences corresponded to the lowest energy configuration. Their computations also showed that layer exchange was not unique to strontium and titanium; in fact, it was expected for many different materials systems. With this understanding, scientists can control—on an atomic level—the growth of oxide thin-films.
"What we have here is a new strategy for materials design and synthesis," said Argonne materials scientist and article co-author Dillon Fong. "Our combination of in situ X-ray scattering with computational theory can be extended to other layered materials and structures, even theoretical ones that haven't been made yet because they are challenging to manufacture."
This new strategy gives faster feedback on what growth strategies are best, thus shortening the timeframe to actual manufacture of a new, stable material.
In the future, Argonne wants to make oxide MBE a tool available to APS facility users for synthesis science. "The APS was instrumental in making our findings possible," explained Freeland. "The X-rays gave us the quantitative information we needed to plug into the theoretical framework, which in turn will allow us—and other APS users—to make new materials more efficiently."
Films were grown in the in situ X-ray chamber at Sector 33ID-E of the APS. Calculations were carried out on the Fusion Cluster of Argonne's Laboratory Computing Resource Center at the National Energy Research Scientific Computing Center (NERSC) and on Argonne's Carbon Cluster.
More information: The paper, "Dynamic layer rearrangement during growth of layered oxide films by molecular beam epitaxy," was published in Nature Materials: www.nature.com/nmat/journal/va … t/full/nmat4039.html
Journal information: Nature Materials
Provided by Argonne National Laboratory