Safer, cheaper building blocks for future anti-HIV and cancer drugs
A team of researchers from KU Leuven, in Belgium, has developed an economical, reliable and heavy metal-free chemical reaction that yields fully functional 1,2,3-triazoles. Triazoles are chemical compounds that can be used as building blocks for more complex chemical compounds, including pharmaceutical drugs.
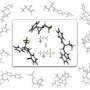
Leveraging the compound's surprisingly stable structure, drug developers have successfully used 1,2,3-triazoles as building blocks in various anti-HIV, anti-cancer and anti-bacterial drugs. But efforts to synthesize the compound have been hampered by one serious hurdle: they depend on harmful heavy metals to work, and this severely limits their biological applications.
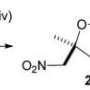
In new experiments reported in the journal Angewandte Chemie, Dr. Joice Thomas, Prof. Dr. Wim Dehaen and their team at KU Leuven's Molecular Design and Synthesis lab confirm for the first time that 1,2,3-triazoles can be synthesized through a metal-free, three-component reaction using readily available ingredients.
"We were able to develop a reaction that provided a good yield, high regioselectivity and easy access to diversely functionalized 1,2,3-triazoles," says corresponding author Prof. Dr. Wim Dehaen. "In other words, the reaction produces plenty of the compounds we're looking for, does so reliably without unwanted or unexpected outcomes, and does this in a way that makes it easy for us to isolate the compound. This makes our method highly desirable."
"Moving forward, we will focus on expanding the chemistry developed here to other new reactions while also exploring their possible applications in pharmaceutical as well as supra-molecular sciences," says lead author Dr. Joice Thomas.
Journal information: Angewandte Chemie
Provided by KU Leuven