'Work environment' affects protein properties
The function of proteins, which fulfil various tasks inside the cells, is often analysed in aqueous buffer solutions. However, it is not known, for example in case of pharmaceutical studies, if they work in the same way in those solutions as in their natural environment: the cytoplasm is highly crowded with biomolecules, organic and inorganic substances.
Under the tutelage of Junior Professor Dr Simon Ebbinghaus, researchers from Bochum have demonstrated that the water surrounding the dissolved substances inside the cell plays a crucial role with regard to protein stability, which has frequently been neglected in the past. The researchers have published the results of their study, gained by means of simple model systems and thermodynamic analyses, in the Journal of the American Chemical Society (JACS). The results have been obtained following a collaboration under the umbrella of the Excellence Cluster RESOLV. The researchers were, moreover, presented with a Hot Topic Poster Award at this year's Bunsentagung in Hamburg.
Proteins' work environment more viscous than egg white
Proteins are among the most important and most-studied biomolecules in biochemical research. They fulfil structure-relevant tasks and are the cells' molecular machines: catalysing chemical reactions, transporting metabolic products and identifying metabolites. Consequently, deficiencies of protein function often result in severe disorders, e.g. Huntington's and Alzheimer's disease. The proteins' natural work environment inside the cell is a highly crowded solution (more viscous and more highly concentrated than egg white), consisting of various macromolecules as well as small organic and inorganic substances. In order to study protein function using modern analytical methods, researchers often purify proteins in vitro and study them in diluted aqueous solution. However, it often cannot be precisely ascertained to what extent the experimental results reflect the actual function within the cellular environment.
Theory: in narrow spaces, proteins "tuck in their elbows"
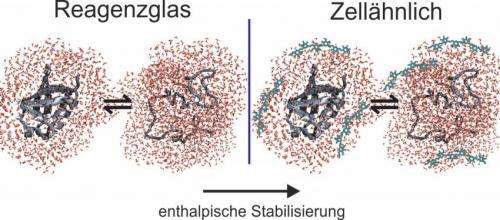
A method frequently used for predicting the effect a densely-packed cellular environment has on proteins is the so-called excluded volume theory. It can be best explained using the example of an everyday phenomenon. In a crowded lift or on a crowded train, everybody attempts to avoid direct contact with their neighbours and to assume as compact a posture as possible (e.g. by tucking in their arms). According to the excluded volume theory, the principle of mutual repulsion can be applied to proteins in a densely-packed cellular environment. They assume a compact structure. As a rule, their biologically active status is at the same time their most compact status, which is why the excluded volume theory predicts a stabilisation of the biologically active status.
The study demonstrates that there are more factors at work than just mutual repulsion
Deploying various solvent additives, such as biomacromolecules, sugars and salts, the RUB researchers have imitated various cellular conditions and analysed the way they affect the model protein ubiquitin. They demonstrated that the proteins' behaviour is affected by more than just mutual repulsion between protein and solvent additive. In thermodynamic studies, different stabilisation and destabilisation mechanisms were discovered. Contrary to the expected so-called entropic stabilisation – based on the excluded volume effect – the researchers observed a so-called enthalpic stabilisation of ubiquitin in the presence of macromolecules, sugars and salts. Enthalpic stabilisation is directly correlated to the strengthening of the chemical bonds in the biologically active status and cannot be explained away with the protein's more compact shape being purely volume-based.
Water as mediator between protein and dissolved substances
The researchers attribute the enthalpic stabilisation phenomenon to a water-mediated process: protein and solvent additive do not interact directly, but following a modification of the water properties of the solvent additives' hydration water, the hydrogen bridge bonds in the protein's biologically active status are optimised. The project was financed by the Returnee Programme of the Ministry for Innovation, Science and Research of North Rhine-Westphalia, the Excellence Cluster RESOLV and the Chemical Industry Association (Verband der Chemischen Industrie e.V., VCI).
More information: Michael Senske, Lisa Törk, Benjamin Born, Martina Havenith, Christian Herrmann, Simon Ebbinghaus (2014): Protein stabilization by macromolecular crowding through enthalpy rather than entropy. Journal of the American Chemical Society. J. Am. Chem. Soc., DOI: 10.1021/ja503205y
Journal information: Journal of the American Chemical Society
Provided by Ruhr-Universitaet-Bochum



















