Student research leads to method for developing clean hydrogen fuel

(Phys.org) —Recycling waste materials into new products is a common method for sustaining a green environment, but it isn't only limited to reusing old cans, plastics, and paper. In chemistry, scientists use discarded materials to create renewable sources of energy.
A pair of Rutgers University–Camden students has found a new family of functional materials for the production of clean hydrogen fuel through photocatalysis, a process that uses sunlight or ultraviolet light to drive chemical reactions.
"We're taking something that is discarded as waste and turning it into something useful," says Sean Taylor, a senior chemistry major at Rutgers–Camden and Sterling High School graduate from Stratford.
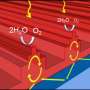
For this research project, Taylor and fellow senior chemistry major Mihir Mehta reuse glycerol, a sustainable compound discarded as waste when vegetable oils are used to create biofuel. The two students mix the glycerol with water and a specially prepared titanium dioxide photocatalyst. The hydrogen resulting from the mixture is an ideal fuel to meet the need for sustainable and renewable sources of energy.
"It becomes an alternative energy source," Taylor notes. "Photocatalysis has been widely studied for a long time and this is our own spin on it. It's like a puzzle and we're trying to build all of the pieces that will fit together to create a bigger picture."
Alexander Samokhvalov, an assistant professor of chemistry at Rutgers–Camden who is advising Taylor and Mehta on the project, says the specially prepared titanium dioxide includes a combination of nitrogen and an inexpensive metal such as nickel or copper.
"In the past, an expansive platinum group metal or gold was normally needed to achieve the high conversion of glycerol to hydrogen," Samokhvalov says.
Once exposed near ultraviolet light that is present in sunlight, the catalytic compound works to transform glycerol and water to hydrogen.
"Our research allows us add yet another method of fuel production that harnesses sunlight," says Mehta, a Delran resident and Cinnaminson High School graduate.
Samokhvalov says by further developing this method, scientists can continue to find ways to develop clean hydrogen fuel "by using the active and affordable functional materials, and use hydrogen as a clean energy source."
"This method allows a broad avenue toward the discovery of similar highly active photocatalysts for the production of clean hydrogen fuels using sunlight," Samokhvalov says.
Taylor, Mehta, and Samokhvalov recorded the results of their experiments in a research paper titled "Production of Hydrogen by Glycerol Photoreforming Using Binary Nitrogen-Metal-Promoted N-M-TiO2 Photocatalysis." The paper was published in a recent issue of ChemPhysChem, a European journal of chemical physics and physical chemistry.
"It's a great honor just to have my name on a paper as an undergraduate student," Taylor says. "It's been an amazing experience to have the opportunity to do the kind of research as an undergraduate student that can have a real impact on the scientific community."
Mehta adds, "Working in a research lab as an undergraduate student allows me to learn new techniques outside of the classroom or student labs. It has taught me to solve problems and to find different methods to obtain our goals."
Journal information: ChemPhysChem
Provided by Rutgers University