Broadening the scope for synthesising optically active compounds
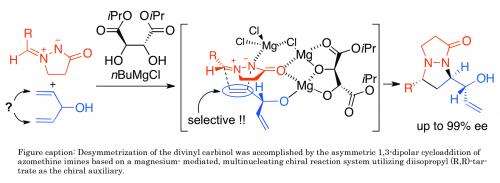
Chiral compounds are increasingly important in chemical manufacturing. They are distinguished by a special kind of asymmetry in their molecular structure. Yutaka Ukaji and colleagues at Kanazawa University have now developed a method for desymmetrising compounds to produce new chiral molecules. The process allows 99% selectivity in the chemicals produced.
The property of chirality is defined by the existence of distinct mirror image geometric arrangements of the constituent parts of a molecule, known as stereoisomers. Just as your right hand cannot be directly superimposed on the left, if the molecule is chiral the mirror images cannot be directly superimposed. Chiral compounds are often described as optically active as one stereoisomer will rotate the plane of incident polarised light to the left and the other will rotate it to the right.
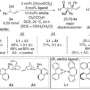
Desymmetrisation methods to produce chiral compounds exist but the range of compounds amenable to the approach remains limited. Ukaji and his colleagues focused on a type of organic compound known as divinyl carbinols – where the vinyl group describes an ethylene molecular group and the carbinol describes an alcohol derived from methanol. Desymmetrisation of divinyl carbinols can provide new optically active alcohol derivatives that contain useful functional groups for further chemical transformations.
The approach developed by the Kanazawa team built on previous work demonstrating an asymmetric 'cycloaddition' reaction where compounds with unsaturated (double, triple etc) bonds combine forming a ring. Their current work demonstrates the reaction on divinyl carbinols with selective production of one mirror image product over the other of over 99%.
They conclude in their report on the work, "This method would be useful for the preparation of optically active nitrogen- and oxygen containing chemicals."
In the meantime, measuring ISG expression patterns in blood and liver samples could provide a useful way of predicting a patient's response to interferon / ribavirin therapy.
More information: Yoshida M, Sassa N, Kato T, Fujinami S, Soeta T, Inomata K, Ukaji Y. "Desymmetrization of 1,4-pentadien-3-ol by the asymmetric 1,3-dipolar cycloaddition of azomethine imines." Chemistry. 2014 Feb 10;20(7):2058-64. DOI: 10.1002/chem.201302889. Epub 2014 Jan 8.
Provided by Kanazawa University