Trees go high-tech: Process turns cellulose into energy storage devices
Based on a fundamental chemical discovery by scientists at Oregon State University, it appears that trees may soon play a major role in making high-tech energy storage devices.
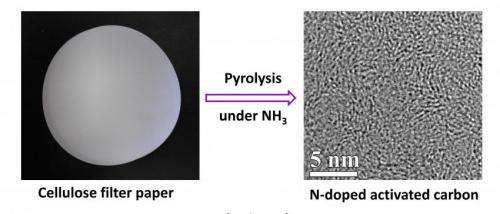
OSU chemists have found that cellulose – the most abundant organic polymer on Earth and a key component of trees – can be heated in a furnace in the presence of ammonia, and turned into the building blocks for supercapacitors.
These supercapacitors are extraordinary, high-power energy devices with a wide range of industrial applications, in everything from electronics to automobiles and aviation. But widespread use of them has been held back primarily by cost and the difficulty of producing high-quality carbon electrodes.
The new approach just discovered at Oregon State can produce nitrogen-doped, nanoporous carbon membranes – the electrodes of a supercapacitor – at low cost, quickly, in an environmentally benign process. The only byproduct is methane, which could be used immediately as a fuel or for other purposes.
"The ease, speed and potential of this process is really exciting," said Xiulei (David) Ji, an assistant professor of chemistry in the OSU College of Science, and lead author on a study announcing the discovery in Nano Letters, a journal of the American Chemical Society. The research was funded by OSU.

"For the first time we've proven that you can react cellulose with ammonia and create these N-doped nanoporous carbon membranes," Ji said. "It's surprising that such a basic reaction was not reported before. Not only are there industrial applications, but this opens a whole new scientific area, studying reducing gas agents for carbon activation.
"We're going to take cheap wood and turn it into a valuable high-tech product," he said.
These carbon membranes at the nano-scale are extraordinarily thin – a single gram of them can have a surface area of nearly 2,000 square meters. That's part of what makes them useful in supercapacitors. And the new process used to do this is a single-step reaction that's fast and inexpensive. It starts with something about as simple as a cellulose filter paper – conceptually similar to the disposable paper filter in a coffee maker.
The exposure to high heat and ammonia converts the cellulose to a nanoporous carbon material needed for supercapacitors, and should enable them to be produced, in mass, more cheaply than before.
A supercapacitor is a type of energy storage device, but it can be recharged much faster than a battery and has a great deal more power. They are mostly used in any type of device where rapid power storage and short, but powerful energy release is needed.
Supercapacitors can be used in computers and consumer electronics, such as the flash in a digital camera. They have applications in heavy industry, and are able to power anything from a crane to a forklift. A supercapacitor can capture energy that might otherwise be wasted, such as in braking operations. And their energy storage abilities may help "smooth out" the power flow from alternative energy systems, such as wind energy.
They can power a defibrillator, open the emergency slides on an aircraft and greatly improve the efficiency of hybrid electric automobiles.
Besides supercapacitors, nanoporous carbon materials also have applications in adsorbing gas pollutants, environmental filters, water treatment and other uses.
"There are many applications of supercapacitors around the world, but right now the field is constrained by cost," Ji said. "If we use this very fast, simple process to make these devices much less expensive, there could be huge benefits."
More information: Study: Pyrolysis of Cellulose under Ammonia Leads to Nitrogen-Doped Nanoporous Carbon Generated through Methane Formation, pubs.acs.org/doi/abs/10.1021/nl500859p
Journal information: Nano Letters
Provided by Oregon State University